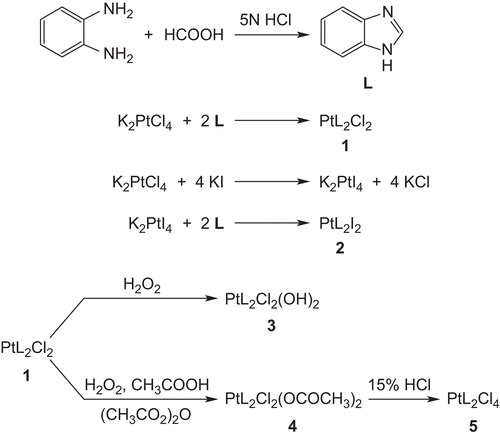
Figures & data
Table 1. IC50 (μM) values of Pt(II) and Pt(IV) complexes on selected human tumor cell lines.
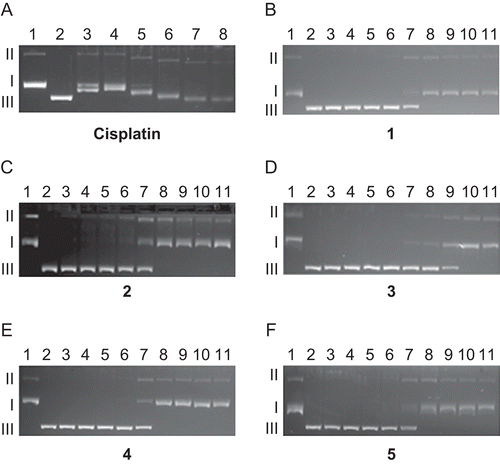
Figure 1. Electrophoretograms relating to the interaction of pBR322 plasmid DNA with increasing concentration of cisplatin and 1–5. Lane 1 in both electrophoretograms contains untreated pBR322 plasmid DNA to serve as a control. For cisplatin, lane 2: 0.625 µM, lane 3: 1.25 µM, lane 4: 2.5 µM, lane 5: 5 µM, lane 6: 10 µM. For 1–5, lane 2: 0.625 µM, lane 3: 1.25 µM, lane 4: 2.5 µM, lane 5: 5 µM, lane 6: 10 µM, lane 7: 20 µM, lane 8: 40 µM, lane 9: 80 µM, lane 10: 160 µM. Roman numerals I and II indicate form I (covalently closed circular) and form II (open circular) plasmids, respectively.
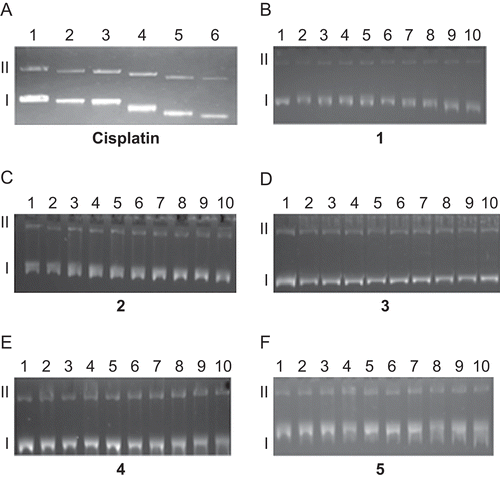
Figure 2. Electrophoretograms relating to the incubated mixtures of pBR322 plasmid DNA and increasing concentration of cisplatin and 1–5 followed by BamH1 digestion. For both cisplatin and 1–5, lane 1 is untreated and undigested with BamH1, lane 2 is untreated pBR322 plasmid DNA but digested with BamH1. For cisplatin, lane 3: 0.625 µM, lane 4: 1.25 µM, lane 5: 2.5 µM, lane 6: 5 µM, lane 7: 10 µM, lane 8: 20 µM. For 1–5, lane 3: 0.625 µM, lane 4: 1.25 µM, lane 5: 2.5 µM, lane 6: 5 µM, lane 7: 10 µM, lane 8: 20 µM, lane 9: 40 µM, lane 10: 80 µM, lane 11: 160 µM. Roman numerals I, II, and III indicate form I (covalently closed circular), form II (open circular), and form III (linear) plasmids, respectively.