Figures & data
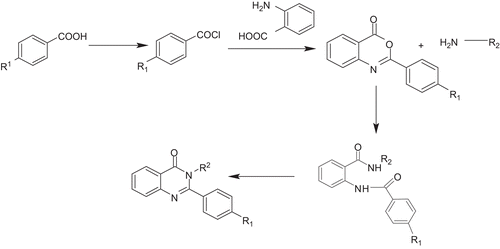
Scheme 1. General schematic synthesis of 2,3-disubstituted 3H-quinazolin-4-ones. Reagents and conditions: (a) thionyl chloride, DMF, reflux; (b) anthranilic acid, pyridine (dry), stir; (c) primary amine, pyridine/benzene, reflux 2–3 h; (d) triethylorthoacetate, para-toluenesulfonic acid (PTSA), primary amine, stir, r.t.; (e) primary amine, ethanol, reflux 2 h.
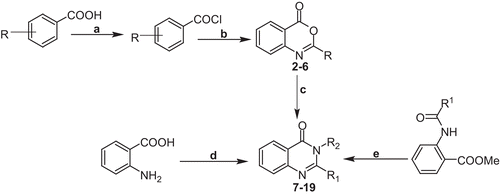
Table 1. Synthesized 2,3-disubstituted quianzolin-4(3H)-ones 7–19 (see ).
Table 2. Antibacterial activities (mm) at 1 mg/mL.
Table 3. Antibacterial activities (MIC) of active compounds 9, 11, 12, and 13.
Table 4. Antifungal activities (% inhibition) at 200 μg/mL.
Table 5. Antifungal activities (MIC) of compounds 7, 8, 10, 12, and 18.
Table 6. Leishmanicidal activities (IC50 values) of compounds 7–19.