Figures & data
Figure 1. Chemical structures of lead compound 1 and modified compounds 8a–d with molecular weight (MW), melting point (m.p.), and yield percentage. Ala, l-alanine; Phe, l-3-phenylalanine; Nal, l-3-(2’-naphthyl)alanine.
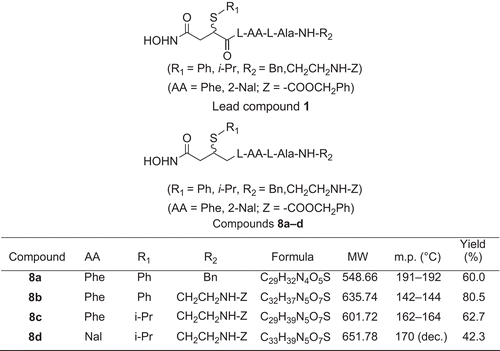
Figure 2. Synthesis of 8a–d. Reagents and conditions: (a) i-PrOH; (b) R1SH/Et3N, benzene; (c) Z-Cl (PhCH2OCOCl); (d) Boc-l-AlaOH, HOBt/DCC, H2NCH2Ph; (e) TFA, Boc-l-AA-OH, HOBt/DCC/Et3N; (f) TFA, HOBt/DCC/Et3N; (g) HONH2/MeOH.
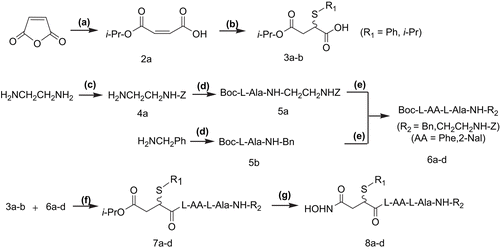
Table 1. Effects of inhibitors on LPS-induced sTNF-α secretion by HL-60.
Figure 3. Content assay of mTNF-α on peritoneal macrophage surfaces of LPS-treated mice by FCM. (A) 0.1 mL PBS (blank control); (B) PBS + Tween-80 (solvent control); (C) Tween-80 containing 1.5 mg of compound 8d; (D) Tween-80 containing 2.5 mg of compound 8d; (A–D) i.p. injection of the above solutions, respectively, followed by intracaudate injection of 0.1 mL solution containing 10 mg d-GalN and 0.4–2 μg LPS; (E) intracaudate injection of 0.1 mL solution containing 10 mg d-GalN and 0.4–2 μg LPS only (positive control).
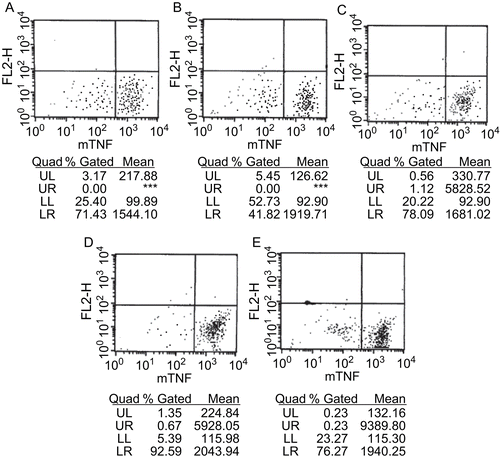
Figure 4. Hematoxylin and eosin (HE) staining of liver and kidney before and after injection of LPS, inhibitor 8d (×200). (A) LPS in liver; (B) LPS and 8d (2.5 mg) in liver; (C) LPS in kidney; (D) LPS and 8d (2.5 mg) in kidney.
Figure 5. Resultant conformation after molecular docking between inhibitor 8c and TACE on SCI Octane 2 graph workstation using advanced Insight II 2003.3L molecular modeling software.
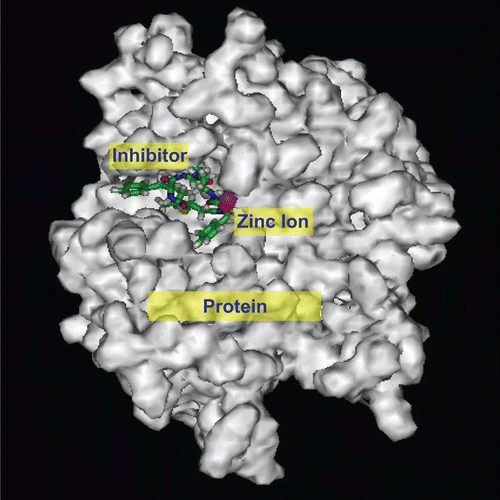
Table 2. Comparison of nonbonding interaction energies between four inhibitors (8a–d) and TACE with results of cell experiments.