Figures & data
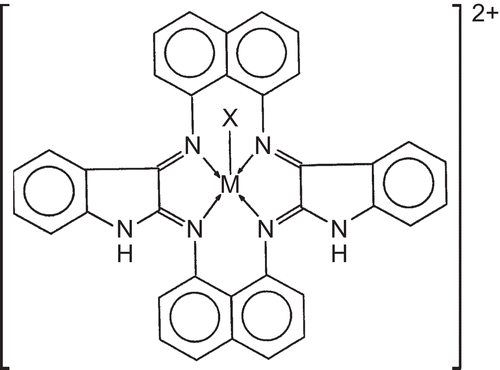
Table 1. Analytical data of trivalent chromium, manganese, and iron complexes derived from 1,8-diaminonaphthalene and isatin.
Table 2. FAB mass spectral data of the trivalent chromium, manganese, and iron complexes derived from 1,8-diaminonaphthalene and isatin.
Table 3. In vitro antibacterial activity of synthesized macrocyclic complexes through agar well diffusion method.
Table 4. Minimum inhibitory concentration (MIC) (in μg/mL) of synthesized macrocyclic complexes by using macrodilution method.
Table 5. Minimum bactericidal concentration (MBC) (in µg/mL) of synthesized macrocyclic complexes by using macrodilution method.
Figure 1. Comparison of MIC (μg/mL) of the synthesized macrocyclic complexes with standard antibiotic. a, Staphylococcus aureus (MTCC 96); b, Bacillus subtilis (MTCC 121); c, Escherichia coli (MTCC 1652); d, Pseudomonas aeruginosa (MTCC 741). Ciprofloxacin, standard antibiotic.
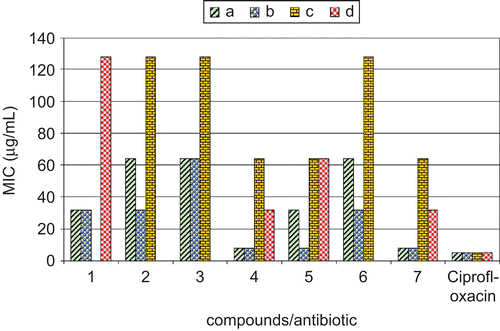
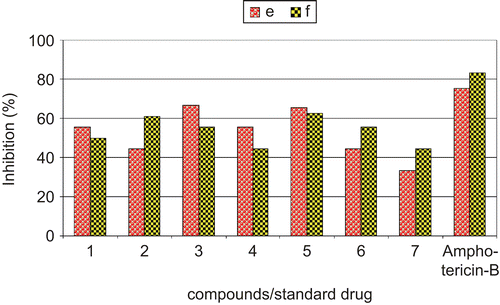
Table 6. In vitro antifungal activities of synthesized complexes through poisoned food method.