Figures & data
Figure 1. Effect of lithium salts on 30 nM RMAK. The 30 nM RMAK was diluted from a 3 µM RMAK stock in 0.01 M potassium phosphate, pH 8 buffer. The activity of RMAK was control, 0.016 eu/mL. The diluted preparations were incubated for 1 h at 25°C and the activities were then determined (see “Methods”).
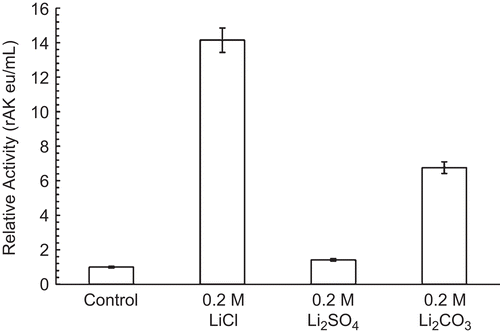
Figure 2. Effect of lithium anions on 30 nM RPFK-1 activity. A 30 nM RPFK-1 was prepared as given in the “Methods” section containing the quantities of lithium salts shown. The preparations were incubated for 1 h at 25°C and the activities were then determined. The RPFK-1 activity of the control was 0.018 ± 0.022 eu/mL.
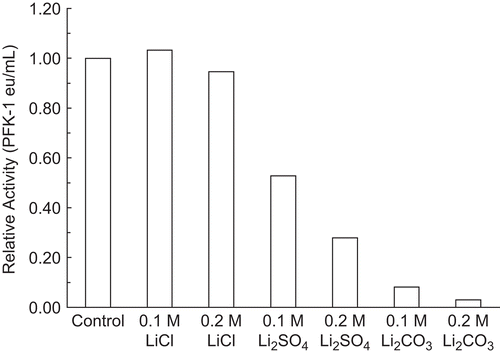
Figure 3. Effect of substrates on 30 nM RPFK-1 inhibition by Li2CO3. A 30 nM RPFK-1 control (▪) with no additions was prepared as given in the “Methods” section containing the quantities of lithium salts shown. The final additions to the control shown are 100 mM F 6-P (•), and 100 mM ATP-Mg (○). In the absence of Li2CO3 average activities were as follows: control, 0.020 eu/mL; plus F 6-P, 0.018 eu/mL; and plus ATP-Mg, 0.015 eu/mL.
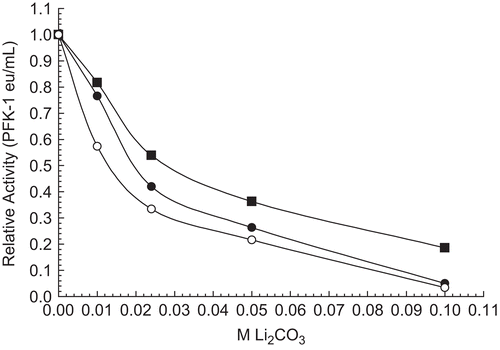
Figure 4. Effect of 5 µM aldolase on PFK-1 inhibition by Li2CO3. A 30 nM RPFK-1 was prepared as given in the “Methods” section containing the quantities of lithium salts shown. The preparations were incubated for 1 h at 25°C and the activities were then determined. The RPFK-1 activity of the control was 0.020 ± 0.002 eu/mL.
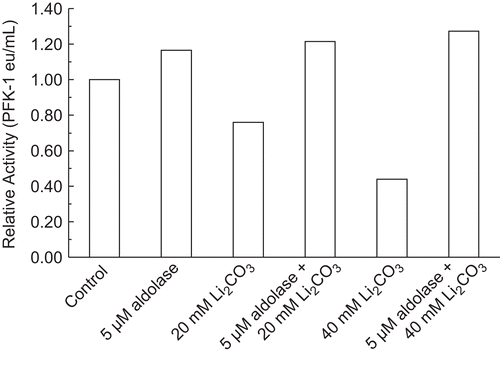
Figure 5. Effect of RPFK-1 concentration on inhibition by 0.075 M Li2CO3. Concentrations of RPFK-1 were prepared by dilutions of a 3 µmol stock as given in the “Methods” containing 0.075 M LiCO3 or not (controls) as shown. Preparations were incubated for 1 h at 25°C and the activities were then determined. The RPFK-1 activity of the control was 0.020 ± 0.002 eu/mL. The RPRK-1 activities of stock controls were as follows: 30 nmol, 0.017 eu/mL; 100 nmol, 0.044 eu/mL; and 200 nmol, 0.29 eu/mL.
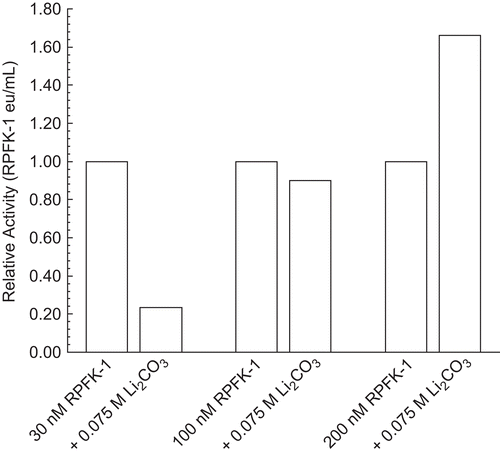
Figure 6. Effect of potassium and sodium salts on 30 nM RPFK-1 activity. The figure is a composite of several experiments using the average of 0.017 eu/mL as the control value for relative activities of 1.00. The 30 nM RPFK-1 solutions were made as given in the “Methods” section, incubated for 1 h; solutions were then made to salt concentrations shown, with an additional 1 h incubation, when RPFK-1 activities were determined. All solutions were maintained at pH 8. The symbols for the salts are as follows: KCl, □; K2CO3, ○; K2HPO4, Δ; NaCl, ▪; Na2CO3, •; and Na2HPO4, ▴.
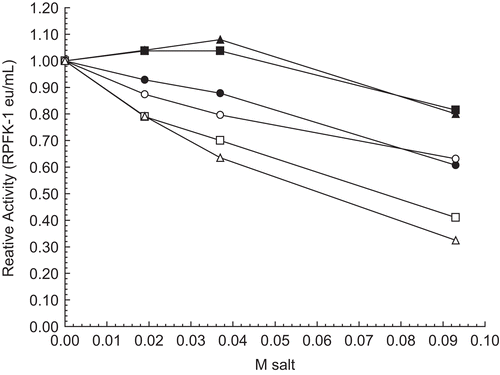
Figure 7. Effect of acetate salts on 30 nM RPFK-1 activity. The figure is a composite of several experiments using the average of 0.010 eu/mL as the control value for relative activities of 1.00. The 30 nM RPFK-1 solutions were made as given in the “Methods” section, incubated for 1 h; solutions were then made to salt concentrations shown, with an additional 1 h incubation, when RPFK-1 activities were determined. All solutions were maintained at pH 8. The symbols for the salts are as follows: sodium acetate, ▪; lithium acetate, •; and potassium acetate, ○.
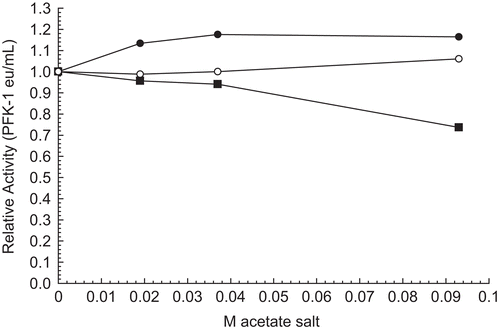
Table 1. Percent inhibition of 30 nM RPFK-1 by 0.1 M monovalent salt.