Figures & data
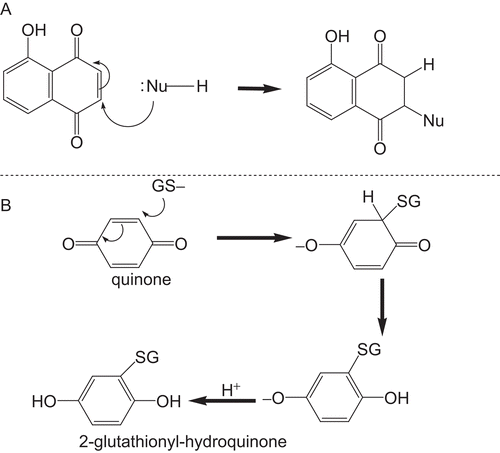
Figure 2. The chemical reactions of CDNB and MCB catalysed by GST P1-1.The monochlorobimane fluorescence assay for GST activity was used to measure the product of conjugation at the excitation wavelength 390 nm and the emission wavelength of 478 nm. The CDNB spectrophotometric assay for GST activity was used to determine the conjugation product 2, at a wavelength of 340 nm.
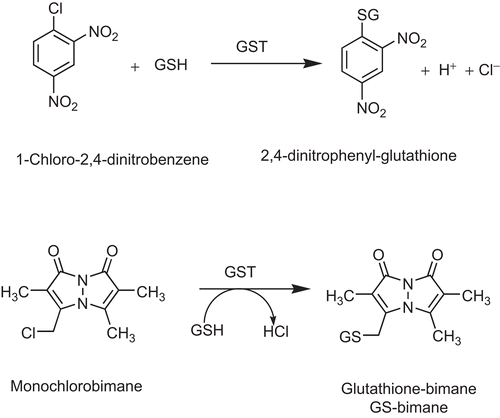
Table 1. Percentage inhibition of GST P1-1 using natural plant compounds at 33 and 100 µM concentration.
Figure 3. Replot of slope (Km/Vmax) and 1/Vmax versus [I] to determine KiGSH and Ki′GSH values of 5,8-dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione (1), which showed mixed type inhibition for GST P1-1 with respect to GSH.
![Figure 3. Replot of slope (Km/Vmax) and 1/Vmax versus [I] to determine KiGSH and Ki′GSH values of 5,8-dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione (1), which showed mixed type inhibition for GST P1-1 with respect to GSH.](/cms/asset/c9238fe4-9201-4a94-9d9f-5b766c5a00f0/ienz_a_526769_f0003_b.gif)
Table 2. The effects of 5,8-dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione (1) on the kinetic properties of GST P1-1 with 1-chloro-2,4 dinitrobenzene as electrophilic substrate.
![Figure 4. Inhibition of GST P1-1 by 5,8-Dihydroxy-1-hydroxymethylnaphtho[2,3-c]furan-4,9-dione (1). The IC50 value is the concentration of inhibitor giving 50% inhibition of enzyme activity. Data are the mean ± standard deviation of quadruplicate experiments each performed twice.](/cms/asset/13aaef8f-a28f-412e-ab9a-fc9590084c09/ienz_a_526769_f0004_b.gif)