Figures & data
Figure 3. The synthesized choline ester derivatives of: 3,4-dimethoxycinnamic acid (1), cinnamic acid (2), caffeic acid (3), rosmarinic acid (4), and trolox (5).
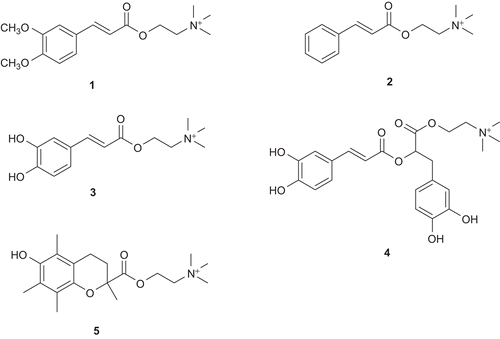
Figure 4. Synthesis of compound 1. Reagents and conditions: (i) SOCl2, reflux; (ii) (MeO)2SO2, K2CO3, THF, reflux; (iii) NaOH, dioxane/H2O, pH 12–14, r.t.; (iv) K2CO3, dioxane/H2O, r.t.; and (v) DMSO, chlorocholine chloride (6), 60°C.
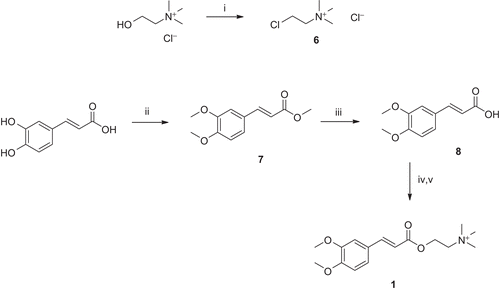
Table 1. Results of AChE inhibition and antioxidant activity (DPPH assay) measured for the compounds in study, some parent compounds and standard AChE inhibitors.
Figure 5. Docking of compound 1 (blue) into the active site of TcAChE (PDB entry 1ACL). The catalytic triad is formed by Ser200, His440, and Glu327, the CAS is formed by Trp84, Phe330, and Glu199, and the PAS located at the entrance of the gorge is formed by Trp279, Tyr70, and Asp72.
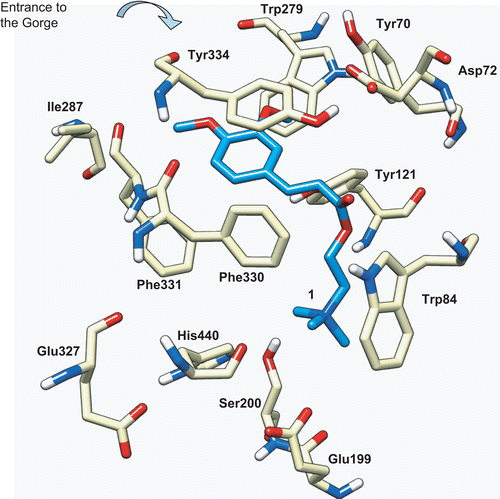
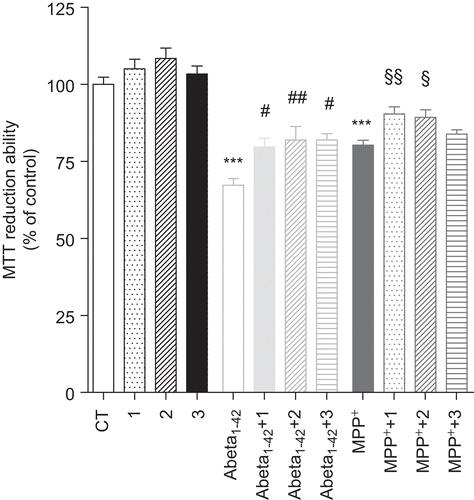
Figure 6. Effect of bifunctional phenolic-choline conjugates (1, 2, 3) on Abeta1–42 and MPP+ toxicity in SH-SY5Y cells. SH-SY5Y cells were treated with Abeta1–42 (1 μM) and MPP+ (1 mM) for 24 h, in the absence or the presence of 5 μM of compounds 1, 2, and 3. Results are expressed as the percentage of SH-SY5Y untreated cells, with the mean ± S.E.M. derived from six different experiments. P < 0.001, significantly different when compared with SH-SY5Y untreated cells; #P < 0.05; ##P < 0.01, significantly different when compared with Abeta1–42 treated cells; §P < 0.05; §§P < 0.01, significantly different when compared with MPP+ treated cells.