Figures & data
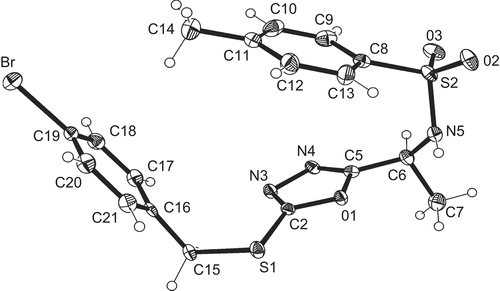
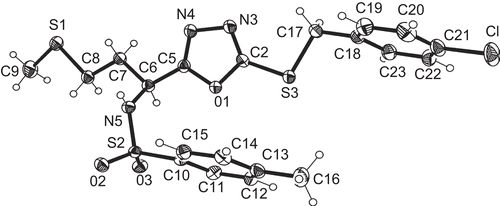
Table 1. Crystallographic data for compounds 5b and 5l.
Table 2. In vitro anti-HIV-1a and HIV-2b of some new sulphonamide derivatives.
Table 3. Calculated value of descriptors.
Table 4. Mulliken charges of the selected atoms.
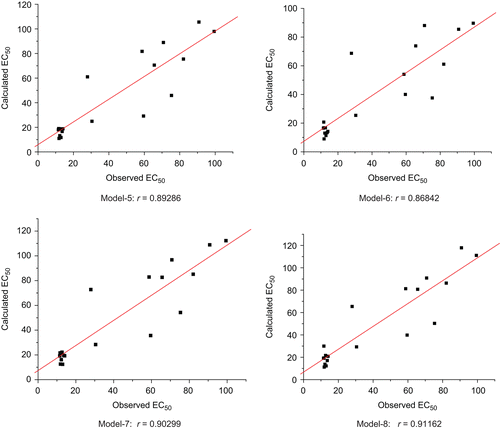
Table 5. Observed and calculated anti-HIV activity (EC50) of given series of compounds.