Figures & data
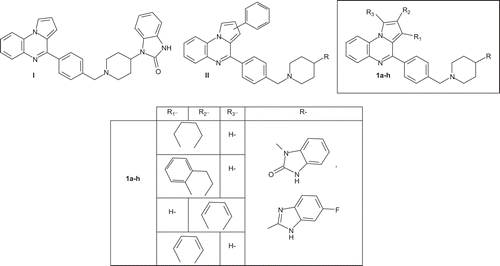
Figure 1. Structure of compounds I, II and new synthesized substituted isoindolo- or indoloquinoxaline derivatives 1a-h.
Scheme 1. Reagents and conditions: (i) DBU, THF/t-BuOH, 50°C; (ii) 1-fluoro-2-nitrobenzene, Cs2CO3, DMF, Δ; (iii) Fe, CH3COOH, Δ; (iv) 1) o-iodotrifluoroacetanilide, K2CO3, CuI, L-proline, DMSO, 80°C; 2) H2O, 60°C (v) POCl3, Δ; (vi) OHC-C6H4-B(OH)2, Pd[P(C6H5)3]4, K2CO3, toluene, EtOH, Δ; (vii) 4-(2-ketobenzimidazolin-1-yl)piperidine or 4-(5-fluorobenzimidazolin-2-yl)piperidine, NaBH3CN, MeOH, Δ.
![Scheme 1. Reagents and conditions: (i) DBU, THF/t-BuOH, 50°C; (ii) 1-fluoro-2-nitrobenzene, Cs2CO3, DMF, Δ; (iii) Fe, CH3COOH, Δ; (iv) 1) o-iodotrifluoroacetanilide, K2CO3, CuI, L-proline, DMSO, 80°C; 2) H2O, 60°C (v) POCl3, Δ; (vi) OHC-C6H4-B(OH)2, Pd[P(C6H5)3]4, K2CO3, toluene, EtOH, Δ; (vii) 4-(2-ketobenzimidazolin-1-yl)piperidine or 4-(5-fluorobenzimidazolin-2-yl)piperidine, NaBH3CN, MeOH, Δ.](/cms/asset/cc5a9a61-5be8-40e4-8359-e6791beb5acd/ienz_a_548326_f0002_b.gif)
Figure 2. The ORTEP drawing of 1,3-dihydro-1-{1-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-ones 1a and 1c with thermal ellipsoids at 30% level.
![Figure 2. The ORTEP drawing of 1,3-dihydro-1-{1-[4-(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]piperidin-4-yl}-2H-benzimidazol-2-ones 1a and 1c with thermal ellipsoids at 30% level.](/cms/asset/78a1dae4-a122-4600-89ea-4f2362461e93/ienz_a_548326_f0003_b.gif)
Scheme 2. Reagents and conditions: (i) NaHSO3, KCN, H2O, 70°C; (ii) CH3COOH, Δ; (iii) KOH, tert-BuOH, 80°C.
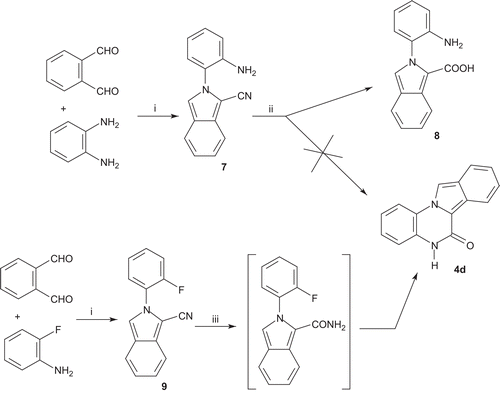
Table 1. In vitro activity of compounds 1a-h on U937, K562, HL60, Jurkat, U266, and MCF7 cells, and cytotoxicity on human peripheral blood mononuclear cells PBMNC + PHA.