Figures & data
Figure 1. (A) 7-Chloro-1-(2,6-difluorophenyl)-1H,3H-thiazolo[3,4-a] benzimidazole (7-Cl-TBZ), (B) 2,3-diaryl-thiazolidin-4-ones, and (C) 4-benzyl/benzoyl-pyridin-2-ones benzylpyridinones.
![Figure 1. (A) 7-Chloro-1-(2,6-difluorophenyl)-1H,3H-thiazolo[3,4-a] benzimidazole (7-Cl-TBZ), (B) 2,3-diaryl-thiazolidin-4-ones, and (C) 4-benzyl/benzoyl-pyridin-2-ones benzylpyridinones.](/cms/asset/ac582be8-514e-4a23-a3ff-dc77a66117a9/ienz_a_548328_f0001_b.gif)
Table 1. Observed and predicted HIV-1 RT inhibitory activity 4-benzyl/benzoylpyridin-2-ones ().
Table 2. Information content of descriptors identified from CP-MLR and GA approaches.
Table 3. Five parameter models for HIV-1 RT inhibitory activity of 4-benzyl/benzoylpyridin-2-ones () from CP-MLR and GA along with statistics.
Table 4. MLR-like PLS models from the combined as well as common descriptors of CP-MLR and GA approaches () for the HIV-1 RT inhibitory activity (−logIC50) of 4-benzyl/benzoylpyridin-2-ones ().
Figure 2. Plots of fraction contribution of MLR-like PLS coefficients (normalized) of the combined and common descriptors of CP-MLR and GA for the HIV-1 RT inhibitory activity of 4-benzyl/benzoyl-pyridin-2-ones; the numbers on the bars refer to the descriptors’ numbers ().
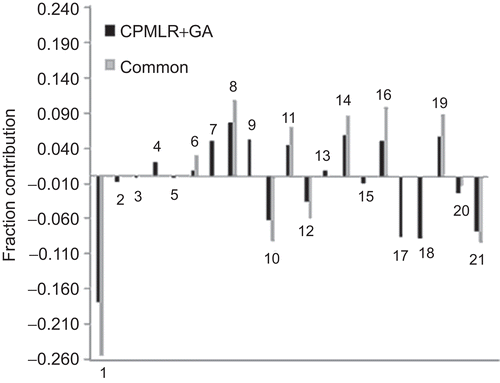
Table 5. ANN Architecture and goodness of fit of HIV-1 RT inhibitory activity of 4-benzyl/benzoylpyridin-2-ones () in training, validation and test sets with five most significant features from PLS in BP-ANN model*.
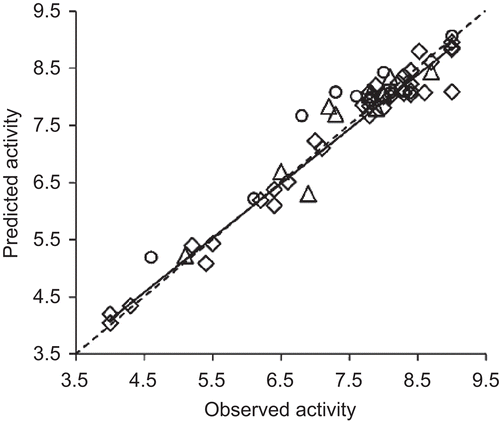
Figure 3. The plots of observed versus predicted activities of 4-benzyl/benzoyl-pyridin-2-ones’ training (open diamonds), validation (open citrcles) and test (open triangles) sets from BP-ANN. The solid line indicates the best fit. The dashed line passing through the origin, making an angle of 45° with the axis, bisects the plot area.