Figures & data
Figure 1. Metabolic pathways for the biosynthesis of DHT in the prostate. The steps that do not require the presence of T are indicated in bold lines.
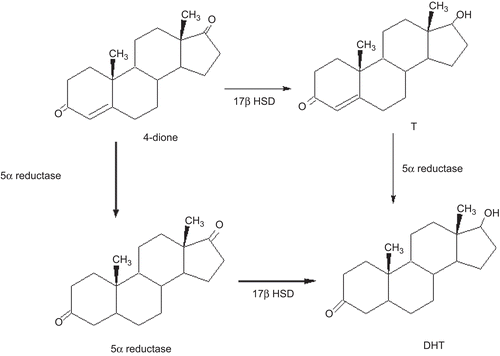
Table 1. DHT and 5α-dione production from different concentrations of labeled T and 4-dione by human prostate homogenates. Effect of increasing concentrations of unlabeled DHT and 5α-dione on the prostatic 5α-reductase activity.
Table 2. T and DHT production from different concentrations of labeled 4-dione by human prostate homogenates. Effect of increasing concentrations of unlabeled 5α-dione.
Figure 2. Enzymatic efficiency (Vmax/Km) was obtained from the rate between maximal velocities (Vmax) of DHT or 5α-dione produced, using different substrates and the Michaelis constants (Km). Vmax/Km was higher when [3H]T+DHT were used as substrate than when T was used as a substrate. Furthermore, Vmax/Km was higher also when [3H]4-dione+5α-dione were used as substrate than when 4-dione was used. However, human prostate 5α-reductase enzyme converts 4-dione to 5α-dione in a similar manner as compared to T to DHT.
![Figure 2. Enzymatic efficiency (Vmax/Km) was obtained from the rate between maximal velocities (Vmax) of DHT or 5α-dione produced, using different substrates and the Michaelis constants (Km). Vmax/Km was higher when [3H]T+DHT were used as substrate than when T was used as a substrate. Furthermore, Vmax/Km was higher also when [3H]4-dione+5α-dione were used as substrate than when 4-dione was used. However, human prostate 5α-reductase enzyme converts 4-dione to 5α-dione in a similar manner as compared to T to DHT.](/cms/asset/1601eac0-733e-427e-8263-aa8fd0cf0eb3/ienz_a_548330_f0002_b.gif)
Table 3. Effect of different compounds as inhibitors of the activity of 5α-reductase.
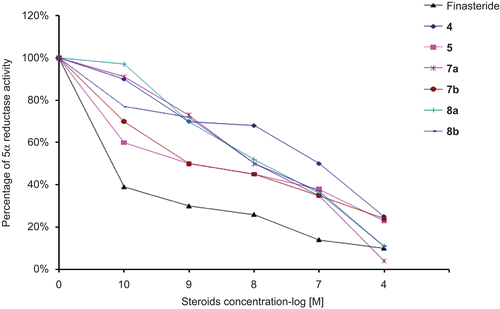
Figure 3. Inhibition plots using different concentrations of the tested steroids against the percentage of activity of 4-dione 5α-reductase. These plots were used for the determination of the concentrations of finasteride as well as the progesterone derivatives 4, 5, 6, 7a, 7b, 8a and 8b required for inhibiting 4-dione 5α-reductase activity by 50%.