Figures & data
Scheme 1. Synthesis of N-[4-chloro-5-R1-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts (12–15). Reagents, conditions and yields: (A) 25% NH4OH / EtOH (1.1 molar equiv.) r.t. 44 h, 73%; (B) anhydrous K2CO3 (excess), dry THF, reflux 24 h, 68%–74%; (C) R2-CH2-X (1.0–1.1 molar equiv.) (X = Br or Cl), water, r.t., 1-2 h, 80%–98%.
![Scheme 1. Synthesis of N-[4-chloro-5-R1-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts (12–15). Reagents, conditions and yields: (A) 25% NH4OH / EtOH (1.1 molar equiv.) r.t. 44 h, 73%; (B) anhydrous K2CO3 (excess), dry THF, reflux 24 h, 68%–74%; (C) R2-CH2-X (1.0–1.1 molar equiv.) (X = Br or Cl), water, r.t., 1-2 h, 80%–98%.](/cms/asset/81c9faad-6eaf-4fe1-b783-66065db5834b/ienz_a_625024_f0001_b.gif)
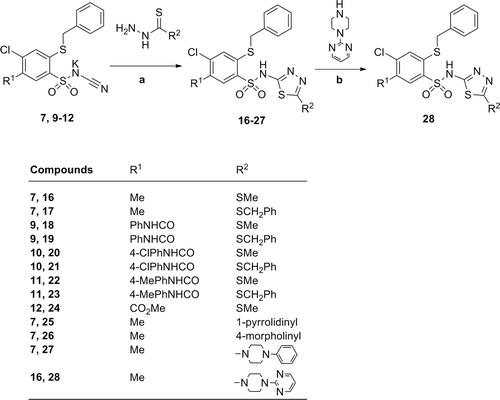
Scheme 2. Synthesis of 2-benzylthio-4-chlorobenzenesulfonamide derivatives (16–28). Reagents and conditions: (A) appropriate hydrazinecarbodithioic acid esters or 1-substituted carbothioic acid hydrazides (1 molar equiv.), glacial acetic acid, reflux 8–40 h; (B) 16 (1 mmol), 2-(piperazin-1-yl)pyrimidine (4.5 mmol), dry toluene, reflux 56 h.
Scheme 3. Synthesis of 4-chloro-2-mercaptobenzenesulfonamide derivatives (29–49). Reagents and conditions: (A) ortho-aminocarboxylate component (1.0–1.1 molar equiv.), glacial acetic acid, reflux 15 h and 12 h at r.t. or reflux 14–17 h; (B) 2-aminophenol or 2-aminothiophenol (1 molar equiv.), glacial acetic acid, reflux 6–7 h, then 12 h r.t.
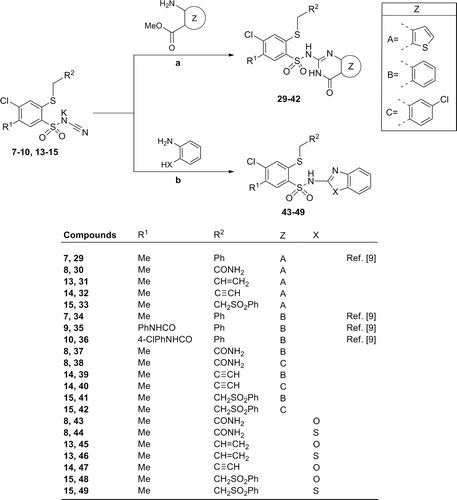
Table 1. Minimal inhibitory concentration (MIC µg/mL) of test compounds 16–24, 26, 28–33.
Table 2. Minimal inhibitory concentration (MIC µg/mL) of test compounds 38–40, 43–49.
