Figures & data
Figure 1. Schematic representation for the three main CA inhibition mechanisms: (A) Sulfonamides (and their isosteres, sulfamate, and sulfamide) substitute the fourth zinc ligand and bind in tetrahedral geometry of the metal ionCitation12; (B) Inorganic anion inhibitors (thiocyanate as an example) add to the metal ion coordination sphere leading to trigonal bipyramidal adductsCitation12; (C) Phenols anchor to the Zn(II) coordinated water molecule/hydroxide ionCitation13; (D) Coumarins (hydrolyzed in situ to 2-hydroxycinnamic acids) occlude the entrance of the active site cavity, interacting both with hydrophilic and hydrophobic amino acid residues. The inhibitor does not interact at all with the catalytically crucial Zn(II) ion which is coordinated by three His residues and a water moleculeCitation10,Citation11.
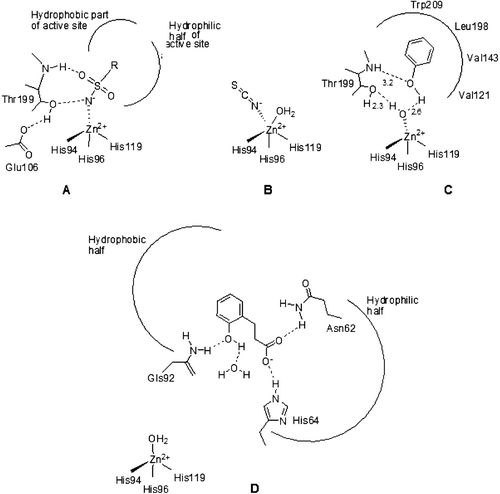
Table 1. KI values (μM) for compound 1–8, EZA, ZNA and AZA of some α-carbonic anhydrase isoforms in human (h) and bovine (b). aRef Citation29.