Figures & data
Figure 2. Purification of bovine PON monitored by SDS-PAGE. The pooled fractions from ammonium sulfate precipitation and hydrophobic interaction chromatography were analyzed by SDS-PAGE (10% separating gel and 3% stacking gel) and revealed by Coomassie Blue staining. Experimental conditions were as described in the method. Lane 1 contained 3 µg of various molecular-mass standards: Bovine serum albumin (66.7 kDa), ovalbumin (45.0 kDa), lactate dehydrogenase (35 kDa), β-lactoglobulin (18.4 kDa), lysozyme (14.4 kDa). Thirty microgram of purified bovine serum PON (lane 2) migrated as a series of three bands with mobilities corresponding to apparent masses of between 38–45 kDa.
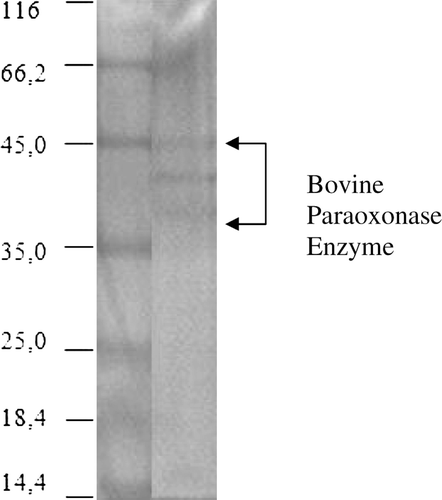
Table 1. Summary of the activity of bovine serum PON1 immobilized using different concentrations of glutaraldehyde.
Figure 4. Comparison of CoCl2, MnCl2 and CuCl heavy metals effect upon the immobilized paraoxonase enzyme activity.
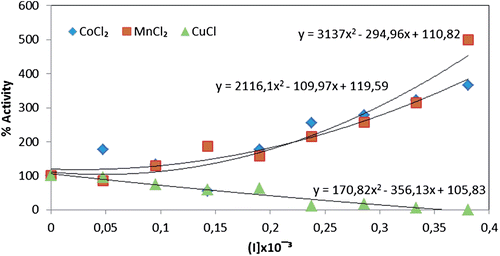
Figure 5. Comparison of CoCl2, MnCl2 and CuCl heavy metals effects upon the free paraoxonase enzyme activity.
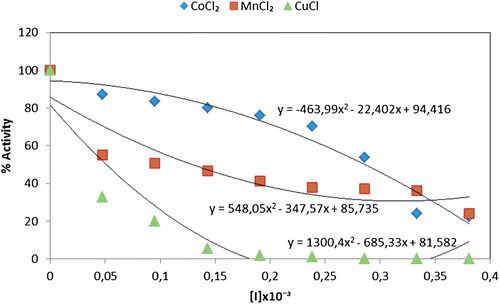
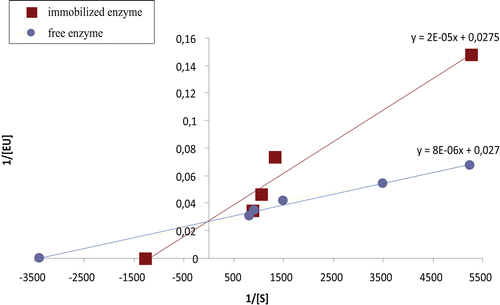
Table 2. Influence of immobilization process on kinetic constants.