Figures & data
Table 1. Synthesis of novel α-oxycarbanilinophosphonates (4) and their anticholinesterase activities.
Figure 1. Stereo image presentation of peripheral site residues of the enzyme and Ser 203. The interacting residues are Trp 286 and Tyr 341. As it can be seen both of the phenyl rings of the compound 4h are interacting with the above-mentioned amino acid residues of the PAS through π–π interactions. The interactions of other residues such as Asp 74 and Tyr 72 are important in the appropriate orientation of the drug.
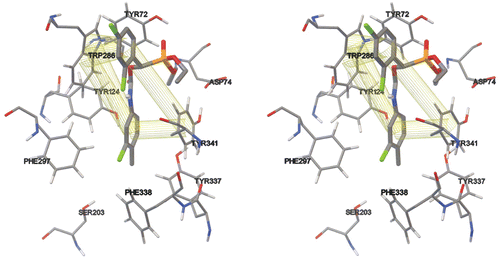
Figure 2. Top view of the surface palatability map of AChE that shows the tri-dimensional position of compound 4h at the peripheral site of the enzyme and its position respect to Ser 203. As it can be seen the orientation of the drug is such that its aromatic ring proximal to the carbamate group has been entered first and the other aromatic ring proximal to the phosphonate group is still outside. It seems that electrostatic interactions between the partially positive phosphorous atom and the negatively charged Asp 74 (shown in dark red colour at the upper right hand corner of the gorge structure) could be of importance in orienting the π–π interactions between the aromatic rings in the compound 4h and aromatic residues in the PAS (i.e. Trp 286 and Tyr 341).