Figures & data
Scheme 1. Synthesis of 6-aminoalkanamido-substituted 2-(1H-indol-2-yl)quinazolin-4(3H)-one derivatives (3a–j). Reagents and conditions: (i) orthoesters, reflux, 3 h; (ii) tryptamine, 115°C, 3 h; (iii) HCI, AcOH, reflux, 1 h; (iv) (v) 10% Pd/C, H2, MeOH, 8 h; (vi) ClCO(CH2)nCl, CH2Cl2, reflux and (vii) HNR2, EtOH, KI, reflux (‘R-’ are shown in ).
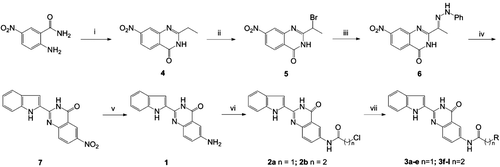
Scheme 2. Synthesis of 4-substituted 2-(1H-indol-2-yl)quinazolin-4(3H)-one derivatives (9a and 9b). Reagents and conditions: (i) POCl3, PhN(CH2CH3)2/Ph, reflux, 6 h and (ii) H2N(CH2)3NR2, EtOH, KI, reflux; (‘R-’ are shown in ).
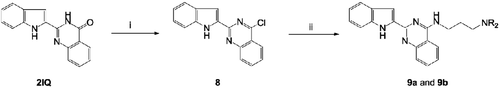
Table 1. In vitro inhibition IC50a and selectivity index of derivatives 3a–j and 9a–b on AChE and BuChE.
Figure 3. Lineweaver–Burk plots of the inhibition kinetics of AChE (A) and BuChE (B) by compound 3h.
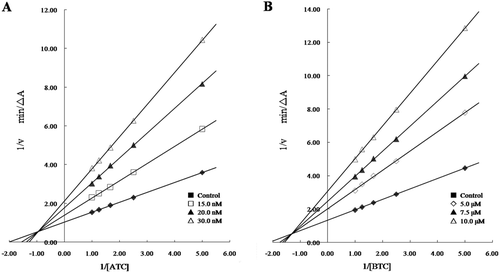
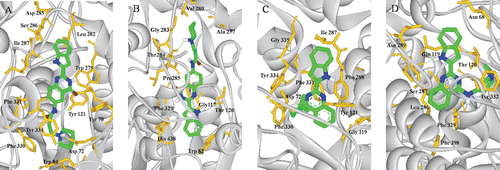
Figure 4. (Colour online) Docking models of compound–enzyme complex. Representations of compound 3h (A, B) and 9a (C, D) interacting with residues in the binding site of TcAChE (A, C) and HuBuChE (B, D). The compounds are rendered in green stick models, and the residues are rendered in golden sticks. Pictures are created with PyMOLCitation27.