Figures & data
Table 1. Description of the main peaks observed in the HPLC–PDA and UHPLC/HR–TOF–MS analyses performed for SAE and LAE.
Figure 1. Chromatograms and UV spectra obtained by LC-PDA for the alkaloids fractions of P. suterella (SAE) and P. laciniata (LAE). Peak 1: UV spectrum characteristic for βC nucleus (further characterized as 1 by co-injection with the isolated standard and by UHPLC/HRTOF-MS and NMR analyses). Peak 2: UV spectrum characteristic of βC nucleus (further characterized as a possible 1 derivative by UHPLC/HR-TOF-MS analysesCitation25). Peak 3: UV spectrum characteristic of THβC MIAs (further characterized as 2 by co-injection with the isolated standard and by UHPLC/HR-TOF-MS and NMR analyses). Peaks 4 and 5: UV spectra characteristic of compounds possessing vallesiachotamine-like nucleus (further characterized as 3 and 4 by GC-MS, UHPLC/HR-TOF-MS and NMR analyses).
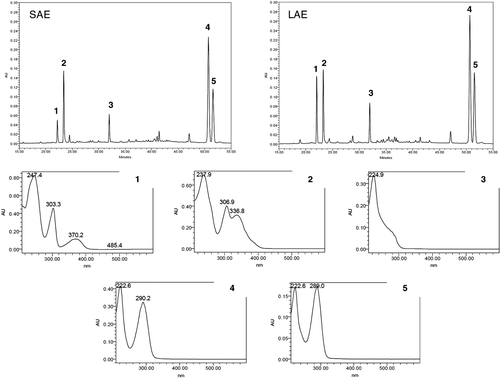
Figure 2. Structures of the alkaloids lyaloside (1), strictosamide (2), vallesiachotamine (3), and isovallesiachotamine (4).
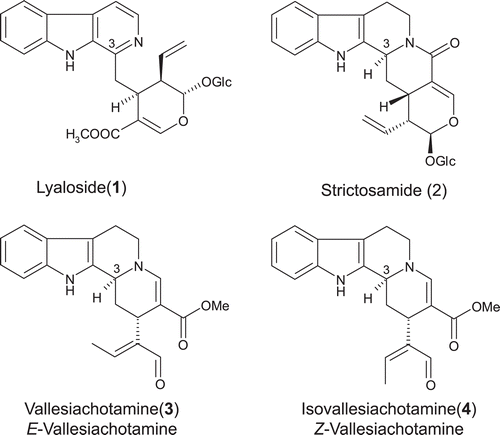
Table 2. MAO-A inhibitory activity of fractions and monoterpene indole alkaloids from P. suterella and P. laciniata.
Table 3. MAO-B inhibitory activity of fractions and monoterpene indole alkaloids from P. suterella and P. laciniata.