Figures & data
Scheme 1. Catalytic and inhibition mechanisms (with zinc ion binders) of α-carbonic anhydrases (CAs) (hCA I amino acid numbering of the zinc ligands). A similar catalytic/inhibition mechanism is valid also for CAs from other classes (β-, γ- and ζ-CAs) but either the metal ion is coordinated by other amino acid residues or a Cd(II) ion is present instead of zinc at the active site.
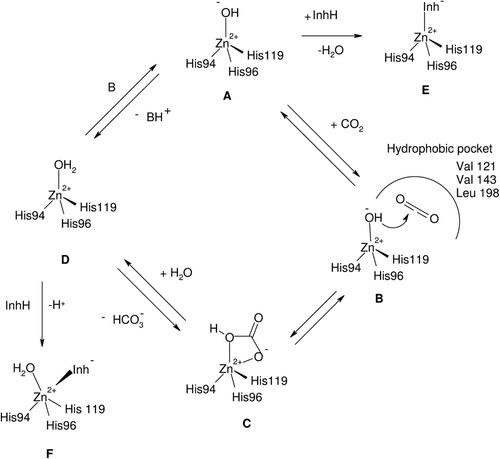
Figure 1. Clinically used/preclinical sulphonamide and sulphamate CAIs 1–15 and compounds developed in the last period (16–27).
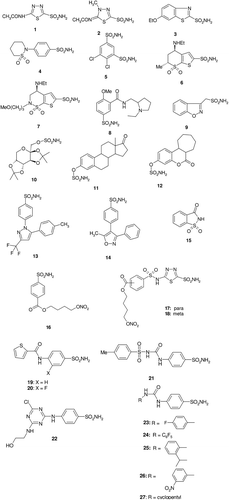
Figure 2. Human carbonic anhydrase (hCA) II in adduct with the sulphonamide incorporating an NO-donating moiety 18, as obtained by x-ray crystallographyCitation49.
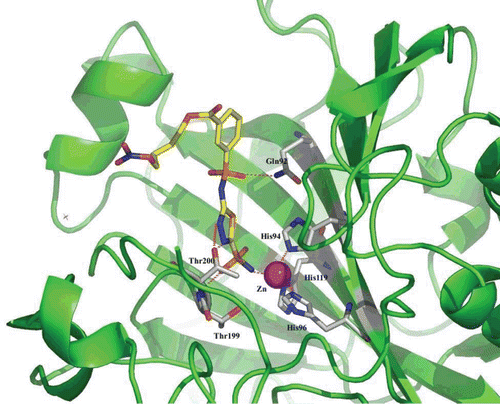
Figure 3. Human carbonic anhydrase (hCA) II complexed with the thienylacetamido benzenesulphonamides 19 (X = H) and 20 (X = F), which illustrate that small changes at the aromatic ring lead to different conformations of the bound inhibitor within the enzyme active siteCitation54.
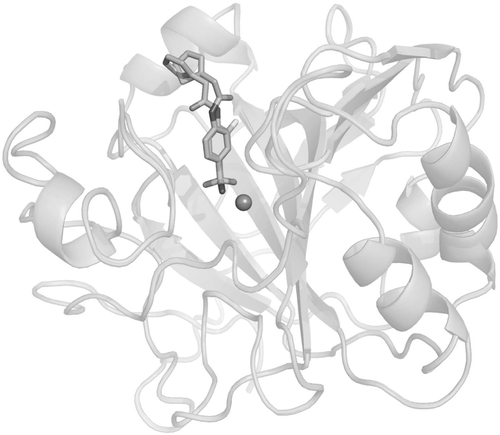
Figure 4. Human carbonic anhydrase (hCA) II complexed with the tosylureido benzenesulphonamide 21 (green) and triazinyl-substituted benzenesulphonamide 22 (blue)Citation56,Citation62 (A); and with five ureido sulphonamides 23–27, compounds 23 (orange), 24 (pink), 25 (yellow), 26 (grey) and 27 (cyan) (B)Citation55.
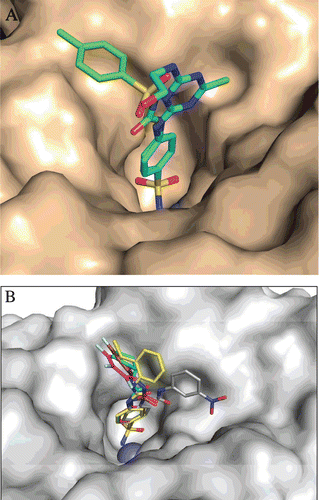
Figure 5. A. Structure of DTCs 28–30. B. Electronic density for the adduct of dithiocarbamate 29 bound within the active site of human carbonic anhydrase (hCA) IICitation59. The zinc ion is shown as the central sphere, and the amino acid residues involved in the binding are evidenced and numbered (hCA I numbering system)Citation59.
Table 1. Inhibition constants of aromatic carboxylates 31–46 against isozymes human carbonic anhydrase (hCA) I and II (α-CA class) and β-CAs Can2 (from Cryptococcus neoformans) and Nce103 (from Candida albicans), for the CO2 hydration reaction, at 20°C. The standard sulphonamide inhibitor acetazolamide (1) was also included as standardCitation71.
Table 2. Carbonic anhydrases (CAs) from pathogenic bacteria cloned and characterised thus far (data for Vibrio cholerae are based on the sequence of a putative CA) and their inhibition studiesCitation8. The genome of many other bacteria contains CAs, which have not yet been cloned and characterised.
Table 3. Inhibition data of the human (h) α-carbonic anhydrase (CA) isoforms hCA I and II and mycobacterial β-CA isoforms mtCA 1 and 3 with dithiocarbamates 47–73 by a stopped-flow, CO2 hydrase assayCitation68.
Table 4. Inhibition data of mycobacterial β-carbonic anhydrase (CA) isoforms mtCA 1–3 with carboxylic acids 74–83 and sulphanilamide 84 by a stopped-flow, CO2 hydrase assayCitation83.