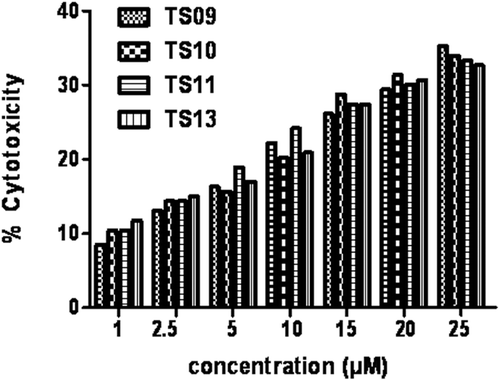
Figures & data
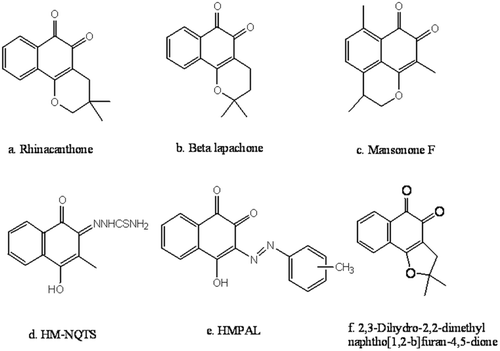
Figure 1. Structures of some potent natural and synthetic anticancer 1, 2-naphthoquinone derivatives.
Scheme 1. General pathway for the synthesis of 4-arylideneamino/cycloalkylidineamino 1, 2-naphthoquinone-2-thiosemicarbazones, reagents & conditions (i) Na2S2O4, stirring at 40–50°C; (ii) FeCl3 in conc. HCl with crushed ice, add with stirring; (iii) sodium azide in water and glacial acetic acid, heated to 40°C; (iv) a. aromatic aldehydes, glacial acetic acid, ethanol/methanol, reflux 6–10 h (iv) b. cyclic ketones, concentrated H2SO4, ethanol/methanol, reflux 8–10 h and (v) a. thiosemicarbazide hydrochloride, ethanol, reflux 4–8 h, (v) b. thiosemicarbazide hydrochloride, ethanol, reflux 10–12 h.
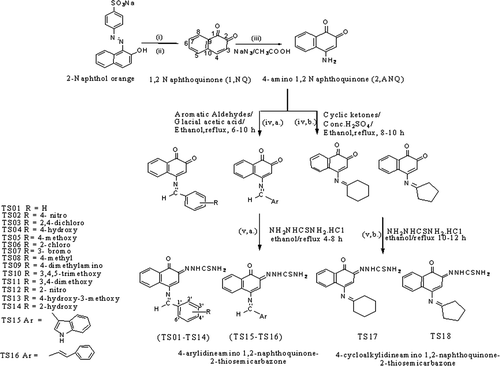
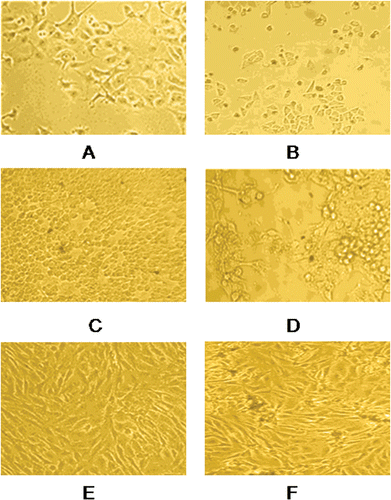
Figure 2. Morphological changes in MCF-7 cells incubated for 48 h, (A) negative control (B) with 5 µM concentration of compound TS10, morphological changes in Hep-G2 cells incubated for 48 h, (C) negative control (D) with 5 µM concentration of compound TS10, morphological changes in MG-63 cells incubated for 48 h, (E) negative control (F) with 5 µM concentration of compound TS10. The cells were observed under phase contrast microscope 100×.