Figures & data
Table 1. Catalytic efficiency and distribution of hCA isoforms.
Table 2. Primary sequence identity (upper right) and number of conserved residues (lower left) among hCA isoforms.
Table 3. Structural main chain r.m.s.d. (Å) among hCA isoforms.
Figure 1. Multiple sequence alignment of the hCA isoforms. Gradients of blue represent sequence conservation from most (dark blue) to least conserved (light blue).

Figure 2. (A) Cartoon representation of superposition of the 11 catalytic isoforms of α-CA, in shades of blue. The histidines (94, 96 and 119) that coordinate the Zn2+ are shown as yellow sticks. (B) Worm representation of main chain r.m.s.d. of the 11 catalytic isoforms. Thicker regions represent more deviation in the main chain. Active site Zn2+ is represented as a magenta sphere. Figure was made using ChimeraCitation110.
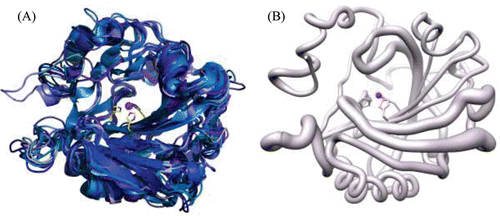
Figure 3. Stick representation of the active site of hCA II (PDB ID 3D92)Citation2 showing key residues that are involved in CO2 binding and proton transfer during catalysis. Residues are as labelled. Active site Zn2+ is shown as a magenta sphere and water molecules involved in proton transfer are shown as red spheres.
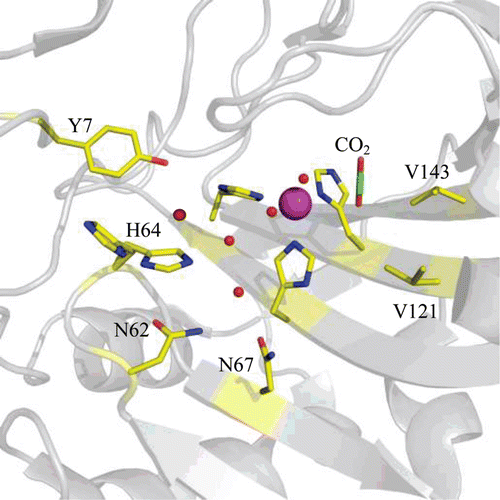
Figure 4. Surface representation of conservation of hydrophobic amino acids among the catalytic α-CAs. The conserved residues are coloured in a gradient of dark (most conserved) to light (least conserved) green. Active site Zn2+ is shown as a magenta sphere. Figure was made using ChimeraCitation110.
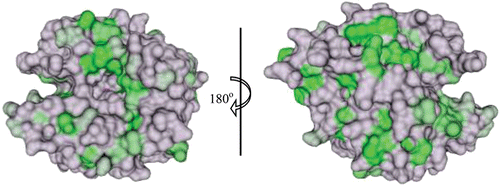
Figure 5. Surface representation of conservation of hydrophilic amino acids among the catalytic α-CAs. The conserved residues are coloured in a gradient of red (negatively charged) and blue (positively charged), from dark (most conserved) to light (least conserved). Active site Zn2+ is shown as a magenta sphere. Figure was made using ChimeraCitation110.
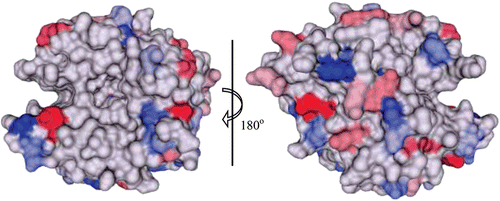
Table 4. Reactions that CAs are believed to be involved in3.
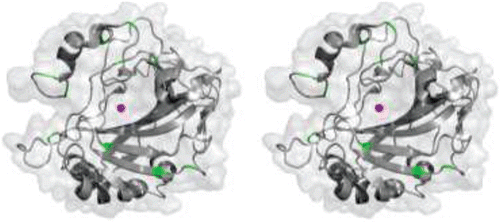
Figure 6. Stereo cartoon-surface representation of hCA II (gray) showing the analogous positions of cysteines (green) present in all the catalytic hCAs. Active site Zn2+ is shown as a magenta sphere.