Figures & data
Figure 2. Structures of the precursor methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate 1, of the nitrodi(hetero)arylamines 2 and of the corresponding aminodi(hetero)arylamines 3.
![Figure 2. Structures of the precursor methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate 1, of the nitrodi(hetero)arylamines 2 and of the corresponding aminodi(hetero)arylamines 3.](/cms/asset/e46ab437-ccb0-4a88-9908-3da14af872bb/ienz_a_777718_f0002_b.jpg)
Figure 3. (A) Scavenging activity on DPPH radicals (RSA), (B) RP (Abs at 690 nm), (C) CLS and (D) TBARS assay using brain homogenized tissue for compounds 3a–c (3a ▴; 3b ▪; 3c •). Results are the mean ± SD of three independent experiments.
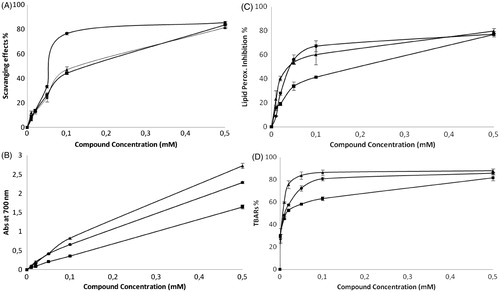
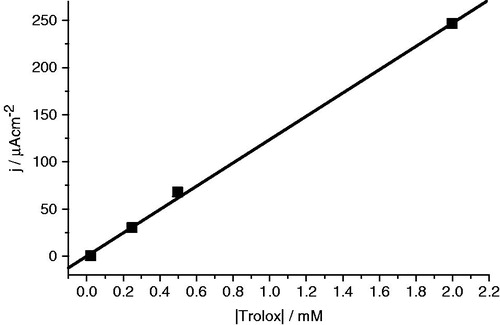
Table 1. EC50 valuesa (µM) obtained for the antioxidant activity of the diarylamines 3a–c and the standard trolox (mean ± SD; n = 3).
Figure 4. Electrochemical responses for 1 mM nitrodi(hetero)arylamines 2, the corresponding aminodi(hetero)arylamines 3 and trolox in 0.1 M TBAP/CH3CN solutions, with Pt electrodes: (left) cyclic voltammogram at 0.1 Vs−1 between −0.5 and 1.2/1.5 V; (right) differential pulse voltammogram obtained with 0.06 V pulse amplitude at 0.02 V.s−1.
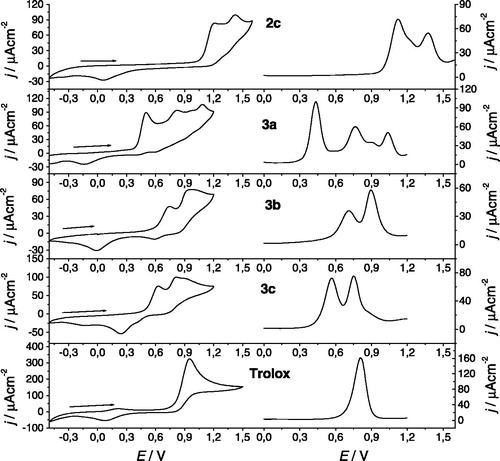
Figure 5. Cyclic voltammograms of 1 mM aminodi(hetero)arylamine 3a in 0.1 M TBAP/CH3CN solution, with Pt electrodes, between −0.5 and 1.2 V at 0.1 Vs−1: (A) first scan; (B) after several scans.
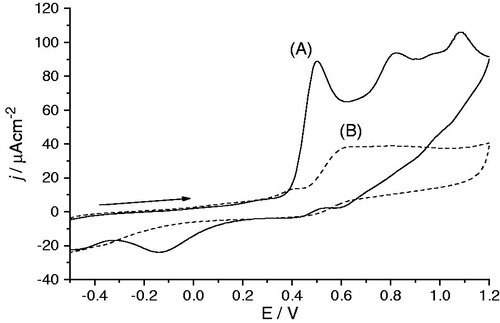
Table 2. Peak potentials obtained for the electrochemical oxidation processes (CV and DPV) of the nitrodi(hetero)arylamines 2, the corresponding aminodi(hetero)arylamines 3, and the standard trolox.