Figures & data
Figure 1. Novel selective MMP-2 inhibitors, where 1 – general structure of aziridine-triazole conjugate; 2 – compound JaZ-30 studied in this work.
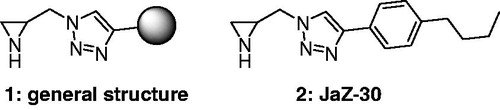
Figure 2. Synthesis of 1-(aziridin-2-ylmethyl)-4-(4-butylphenyl)-1H-1,2,3-triazole (JaZ-30). Details see in Materials and methods section.
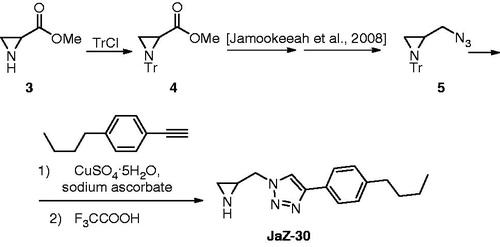
Figure 3. Dose-dependent inhibitory effect of JaZ-30 on MMP-2 catalytic activity in vitro. Relative Fluorescence Units (RFU) at Ex/Em = 328/420 nm were measured in the process of fluorogenic substrate cleavage. Catalytic activity of MMP without inhibitor was taken as 100% of MMP catalytic activity. Results are presented as mean ± SD of triplicates, p = 0.05, statistically significant.
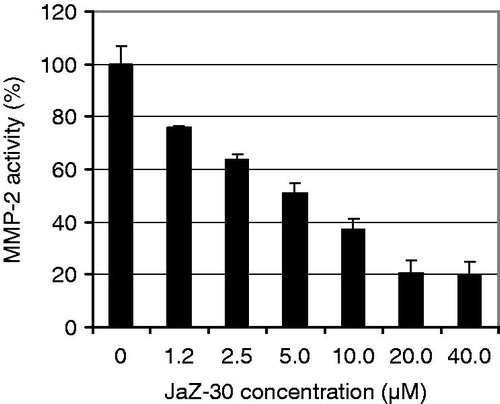
Figure 4. Concentration of VEGF in B16-GFP growth medium. B16-GFP cells were treated with/without 0.5 µM PMA and 10 µM JaZ-30 for 72 h. Details of experiments are in the Materials and methods section. Results are presented as mean ± SD of triplicates, p < 0.05, statistically significant.
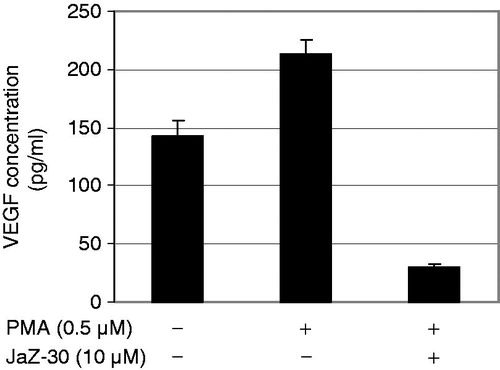
Figure 5. Dose-dependent inhibitory effect of JaZ-30 on the capillary-like network formation of human umbilical vein endothelial cells (HUVECs) in vitro. (A) Images of HUVECs the capillary-like network structure, formed on Matrigel-coated wells surface in 24 h. HUVECs were treated with 5 ng/ml VEGF – control, and indicated concentrations of JaZ-30: 1.2 µM; 2.5 µM; 5 µM, 10 µM and 20 µM; magnification 120 ×. (B) The percentage of angiogenesis quantification by calculation the number of branching points in four images, viewed with an inverted microscope. Results are presented as mean ± SD, p < 0.05, statistically significant.
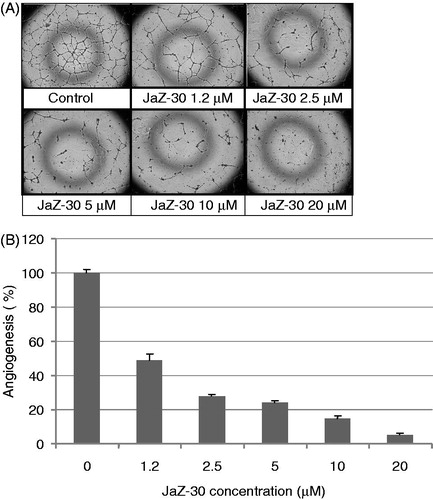
Figure 6. Inhibitory effect of JaZ-30 on the invasion of B16-GFP cells through Matrigel membrane. Cells, invading through Matrigel and Control membrane for 48 h, were quantified by counting live cells in the wells under the insert in fluorescent microscope. Percentage of invasive cells was calculated by a formula indicated in Materials and methods section. Results are presented as mean ± SD of triplicates, p < 0.05, statistically significant.
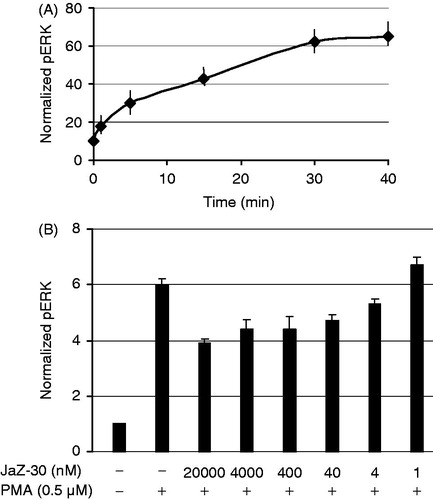
Figure 7. JaZ-30-mediated phosphorylation of ERK1/2 in B16 cells. (A) Kinetics of ERK1/2 phosphorylation in B16 cells treated with 0.5 µM PMA. (B) Inhibition of ERK1/2 phosphorylation (pERK) by different concentrations of JaZ-30 compound. Cells B16 were treated with JaZ-30 for 3 h and then for 30 min with 0.5 µM PMA. Calculation of normalized pERK (phosphorylated ERK1/2 to the total ERK) presented in the section Materials and methods. Each datum represents mean ± standard deviation of triplicate experiments, p < 0.001, statistically significant.