Figures & data
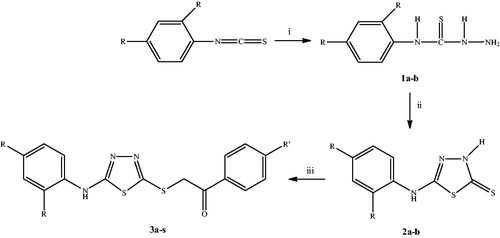
Scheme 1. The synthetic route for the preparation of the thiadiazole derivatives (3a–s). Reagents and conditions: (i) NH2NH2 ċ H2O, ethanol, rt, 5 h; (ii) (1) CS2/NaOH, ethanol, reflux, 10 h; (2) HCl, pH 4–5; (iii) PhCOCH2Br, K2CO3, acetone, rt, 8 h.