Figures & data
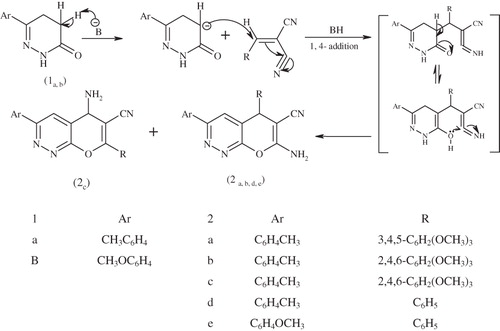
Scheme 1. Mechanism for the synthesis of 2a–e. Reagents and conditions: arylbenzylidine, piperidine, EtOH, reflux 3 h.
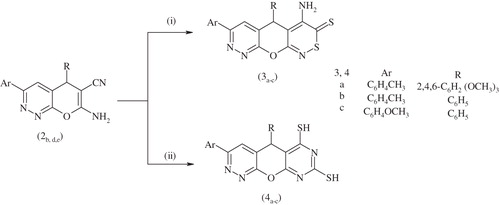
Scheme 2. Synthesis of compounds 3a–c, 4a–c. Reagents and conditions: (i) CS2, piperidine, EtOH, reflux 4 h and (ii) CS2, EtOH, reflux 4 h.
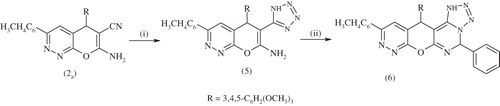
Scheme 3. Synthesis of compounds 5,6. Reagents and conditions: (i) NaN3, NH4Cl, DMF, reflux 5 h and (ii) C6H5CHO, EtOH, piperidine, reflux 2 h.
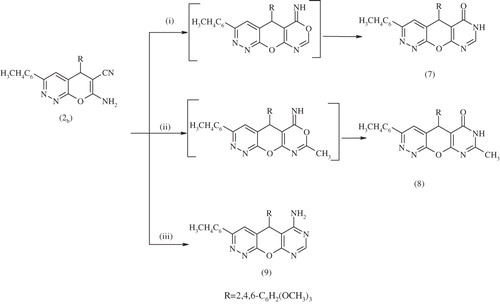
Scheme 4. Synthesis of compounds 7–9. Reagents and conditions: (i) HCOOH, EtOH, reflux 6 h; (ii) (Ac) 2O, EtOH, reflux 6 h and (iii) HCONH2, EtOH, reflux 6 h.
Scheme 5. Synthesis of compounds 10,11. Reagents and conditions: (i) NH2NH2.H2O, EtOH, reflux 3 h and (ii) CH2 (CN)2, piperidine, EtOH, reflux 10 h.
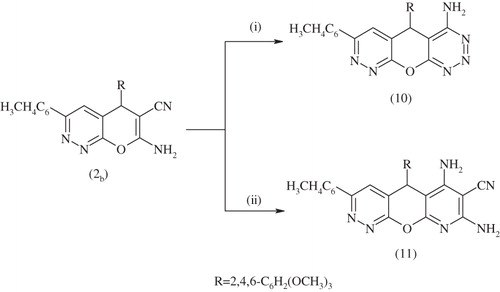
Figure 1. Inhibition zones (mm) and MIC (µ/ml) of compounds 3a–c and chloramphenicol against Klebsiella pneumonia, Escherichia coli, Enterococcusi fecalis and S. aureus strains.
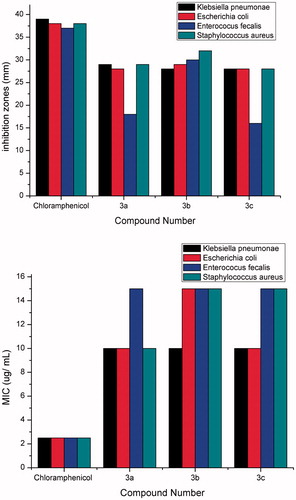
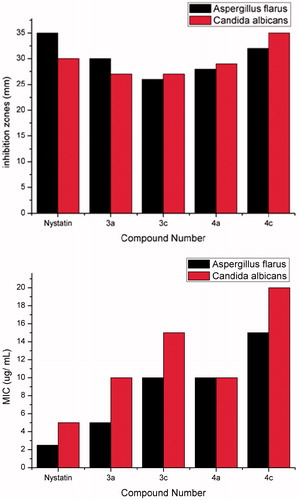
Figure 2. Inhibition zones (mm) and MIC (µ/ml) of compounds 3a,c and 4a,c and Nystatin against Aspergillus flavus and Candida albicans strains.