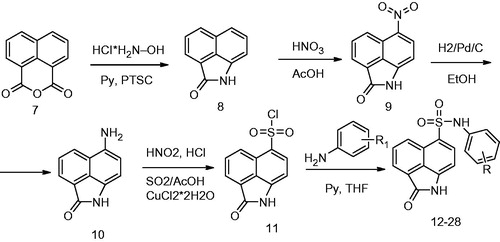
Figures & data
Table 1. Chemical characteristics of synthesized compound.
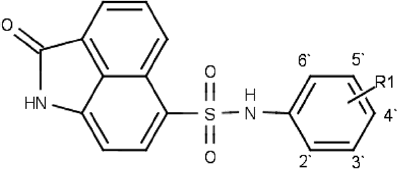
Table 2. Chemical structure and in vitro activities of the naphthostyril derivatives.
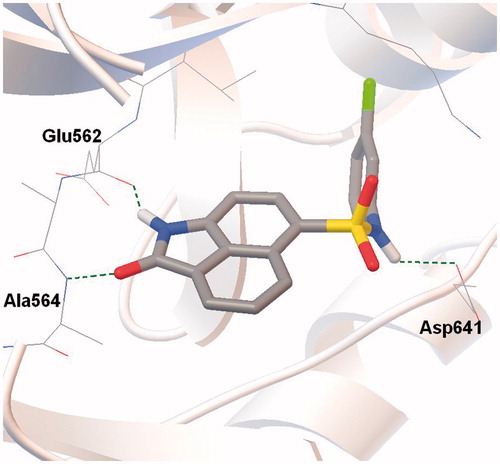
Figure 2. Docking model of compound 1 bound to the ATP-binding site of the FGFR1. Hydrogen bonds marked as dashed lines.