Figures & data
Figure 1. Genomic (slow) and non-genomic (rapid) mechanisms of action of PR complexes (PRA; PRB). Non-genomic signaling requires a membrane progesterone receptor (mPR) that stimulates a cascade which induces CBP phosphorylation and stimulation of co-activators involved in the genomic mechanism.
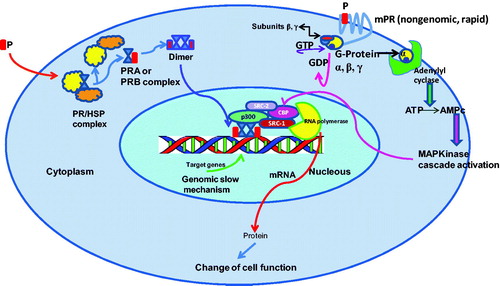
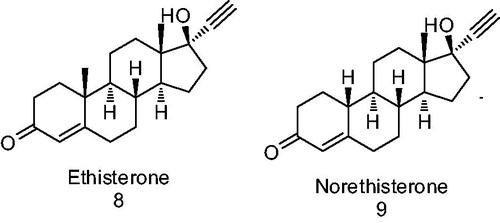
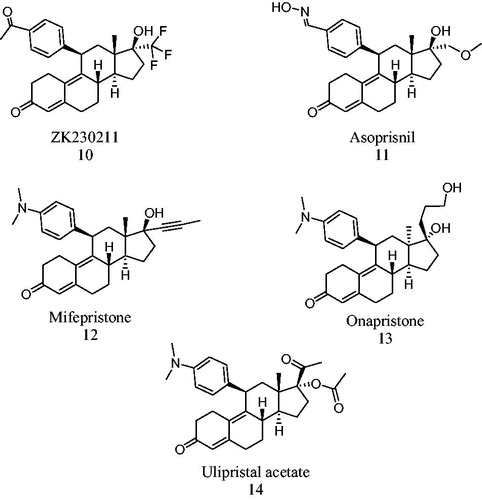
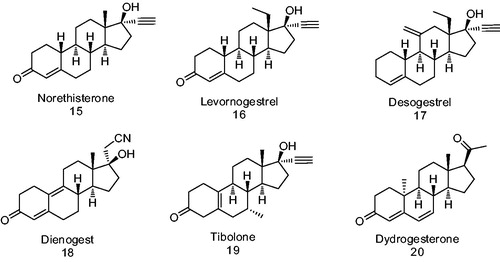
Figure 2. Progestins structurally related to progesterone. 1 – Progesterone, 2 – testosterone, 3 – 17-α-hydroxyprogesterone, 4 – 17α-acetoxyprogesterone, 5 – medroxyprogesterone acetate, 6 – chlormadinone acetate and 7 – cyproterone acetate.
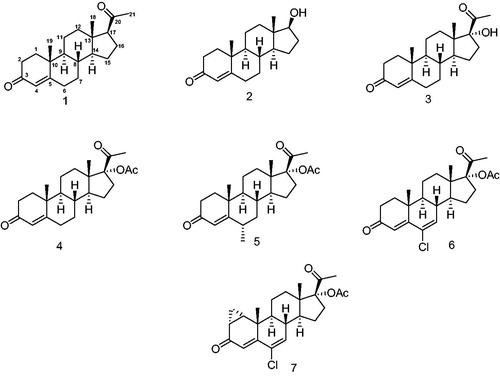
Figure 6. 17-β-Methyl, 16-β-phenyl-D-homoandrost-4,6-diene derivatives having a phenylacetic acid ester at C-17β position and dehydroepiandrosterone derivatives as an antagonists and partial antagonists. 5α-Reductase enzyme IC50 (5α-R IC50) is the concentration of the synthetic steroid to produce 50% inhibition of 5α-R activity.
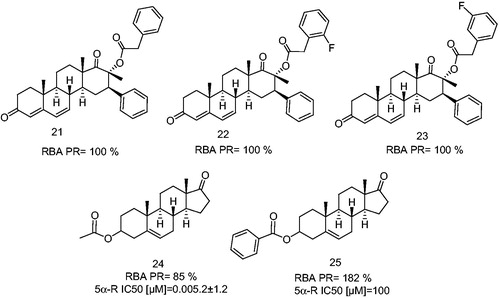
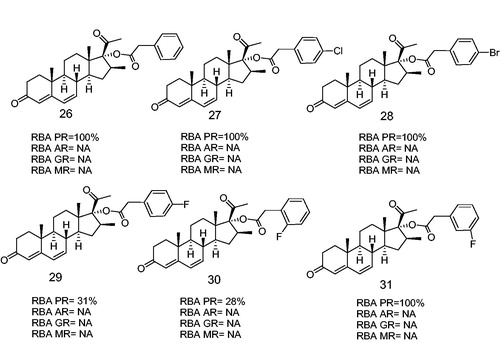
Figure 7. Halogen-substituted phenylacetic acid derivatives antagonists of PR. Androgen receptor (AR), glucocorticoid receptor (GR), mineralocorticoid receptor (MR). RBA, Relative binding affinity; PR, Progesterone receptor; AR, Androgen receptor; GR, glucocoticoid receptor; MR, mineralocorticoid receptor; NA, Non-active.