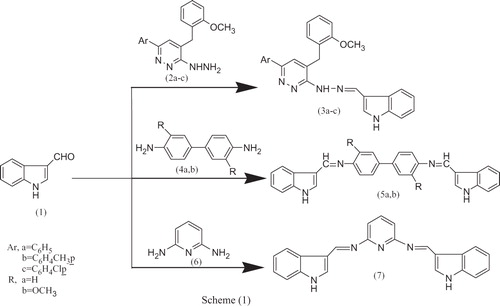
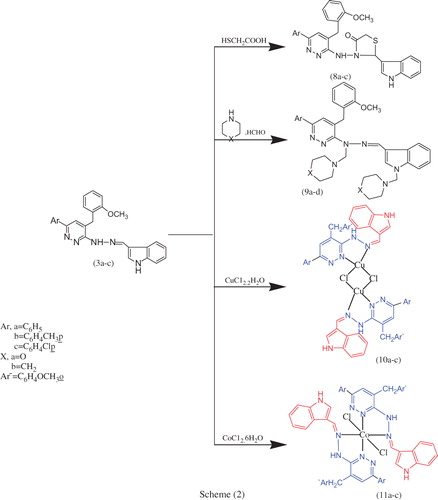
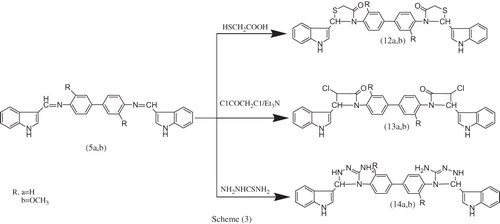
Figures & data
Table 1. Inhibition zone diameter in (mm) as a criterion of antibacterial and antifungal activities of the newly synthesized compounds.
Table 2. Minimum inhibitory concentration (MIC) in µg/ml of the newly synthesized compounds.
Figure 1. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against colon (HCT116) tumor cell line compared to Vinblastine sulphate IC50:9.8.
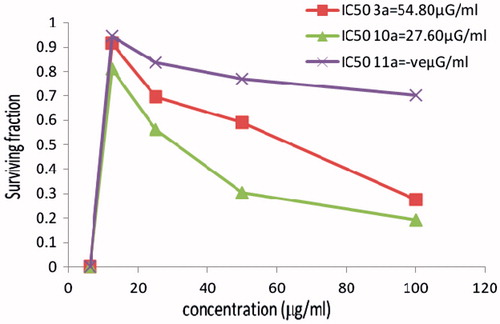
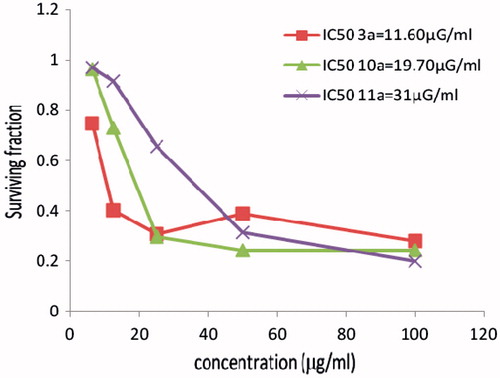
Figure 2. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against breast (MCF7) tumor cell line compared to Vinblastine sulphate IC50:11.6.
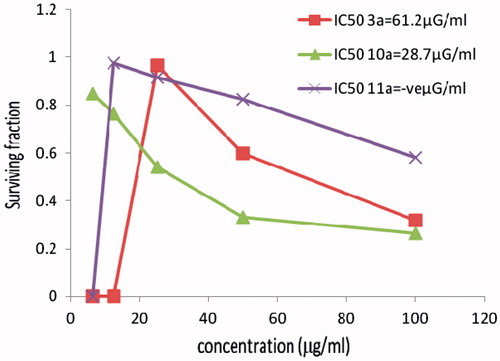
Figure 3. The cytotoxicity data of the activity of compounds (3a, 10a, 11a) against cervix (HELA) tumor cell line compared to Vinblastine sulphate IC50:10.9.