Figures & data
Figure 7. SDS-PAGE of purified human protein kinase CK2 holoenzyme. About 15 µL of purified protein solution (0.25 µg/µL) were separated on a 12.5% acrylamide gel and stained with Coomassie Brilliant Blue G250. At the left, the apparent molecular mass of the marker proteins (lane 1) is given. Lane 2 shows the purified human CK2 enzyme holoenzyme (3.75 µg). The band below the CK2α belongs to the well-known degradation product of the α-subunit CK2α (amino acids 1–335), which is supposed to be enzymatically active and using occurs during purificationCitation33.
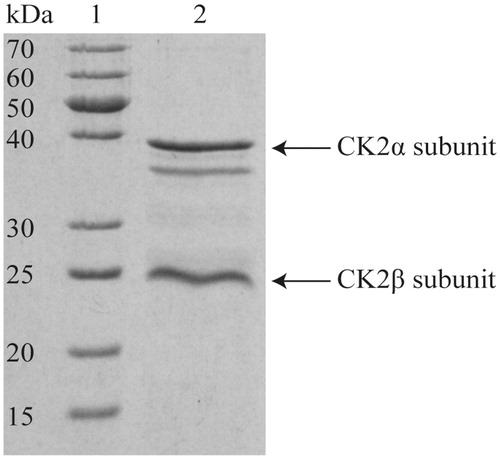
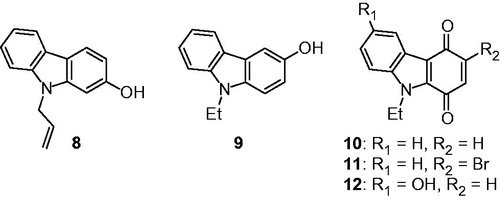
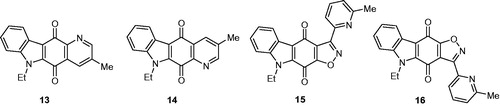
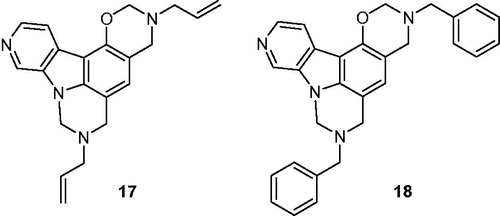
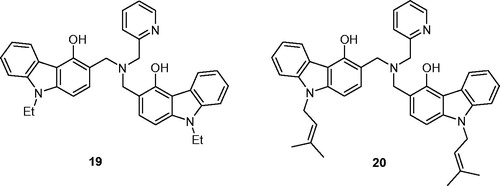
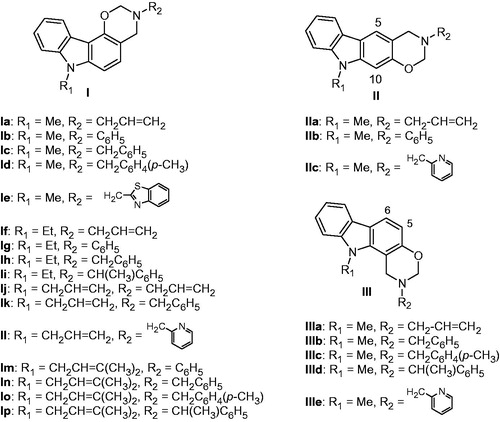
Table 1. Inhibition of human protein kinase CK2 by carbazole derivatives 10–16, Ia–p, IIa–c, and IIIa–e.
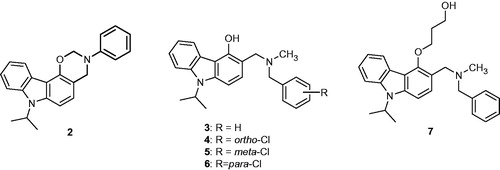
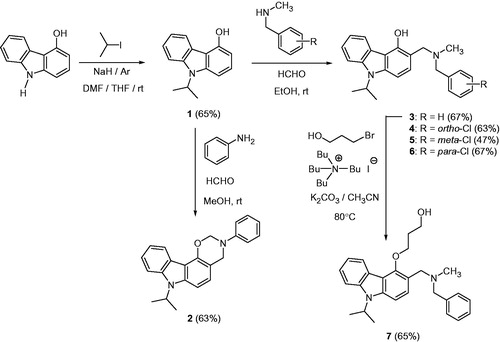
Table 2. Inhibition of human protein kinase CK2 by oxazinocarbazole 2 and aminomethylated carbazoles 3–7.
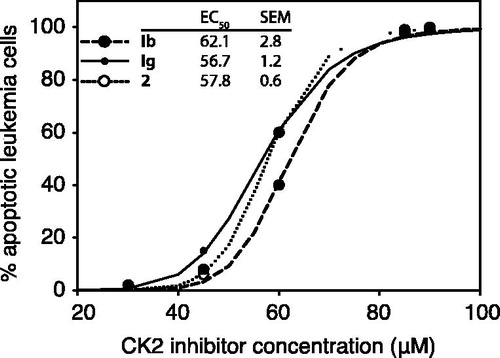
Figure 8. The ability of oxazinocarbazoles to induce IPC-81 leukemia cell death. IPC-81 cells were incubated with various concentrations of the compounds Ib, Ig, and 2, and apoptosis assessed by microscopic evaluation of leukemia cell morphology after 24 h.