Figures & data
Table 1. Some characteristics of the compounds (C1–C10).
Table 2. Some characteristics of the compounds (D1-D38). .
.
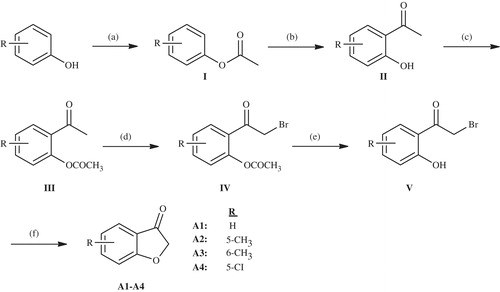
Scheme 1. The synthesis of the benzofuran-3-one derivatives (A1–A4). Reactants/reagents and reaction conditions. a: (CH3CO)2O, reflux, 30 min; b: AlCl3, 160–170 °C; c: (CH3CO)2O, 50–100 °C, 3–4 h; d: Br2, HBr, diethyl ether or acetic acid; e: 10% HCl, reflux; f: CH3COONa, EtOH, reflux.
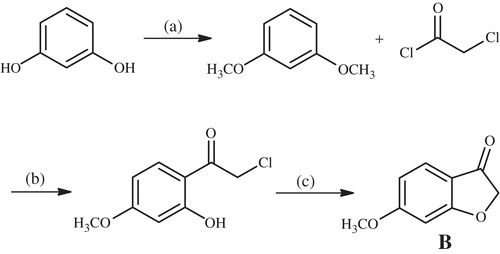
Scheme 2. The synthesis of 6-methoxybenzofuranone (B). Reactants/reagents and reaction conditions. a: (CH3)2SO4, NaOH, acetone, reflux, 4 h; b: AlCl3, CS2, dichloromethane, reflux, 30 min; c: CH3COONa, EtOH, reflux.
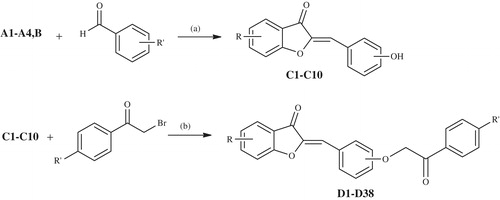
Scheme 3. The synthesis of the final compounds (D1–D38). Reactants/reagents and reaction conditions. a: n-butanol/isobutanol, catalytic HCl, reflux, 1 h; b: acetone, K2CO3, reflux, 6 h.