Figures & data
Figure 2. Time- and concentration-dependent inhibition of TrxR1 by NACC. The natural logarithm of remaining TrxR1 activity is plotted against time. TrxR1 was incubated with various concentrations of NACC (♦, 12.5 μM; ▪, 25 μM; ▴, 50 μM; ○, 100 μM), and aliquots were withdrawn at different time intervals. The data were derived from one of triplicate experiments.
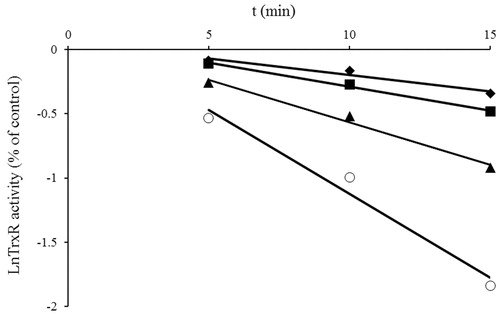
Figure 3. Double-reciprocal plot of the apparent rate constants of inhibition (kapp, slope from ) versus the reciprocal of inhibitor concentration ([I]). The Ki and kinact values were determined to be 80 μM and 0.178 min−1, respectively, based on the method of Kitz and WilsonCitation20.
![Figure 3. Double-reciprocal plot of the apparent rate constants of inhibition (kapp, slope from Figure 2) versus the reciprocal of inhibitor concentration ([I]). The Ki and kinact values were determined to be 80 μM and 0.178 min−1, respectively, based on the method of Kitz and WilsonCitation20.](/cms/asset/f2d3dcc0-d3cf-4f2a-9607-6b4a03f944ab/ienz_a_1016512_f0003_b.jpg)
Figure 4. Determination of the irreversibility of TrxR1 inhibition by NACC via dialysis. The activity of 0.34 unit/mL rat liver TrxR1 was completely inhibited by incubation at room temperature with NACC (1 mM) in the presence of 0.25 mM NADPH for 30 min. Then extensive dialysis was performed in a Slide-A-Lyzer dialysis cassette (Thermo) for 6 h. Aliquots were withdrawn at indicated time intervals, and the remaining TrxR1 activity was determined as described in the TrxR1 assay. No recovery of enzyme activity was observed after 6 h of dialysis for NACC-treated enzyme. The results are presented as the means ± S.D. of three independent experiments. ▪, control; ♦, NACC treated.
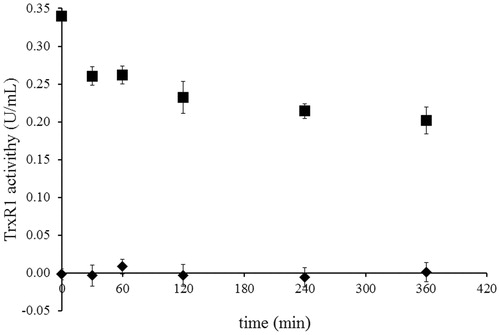
Figure 5. Substrate protection of TrxR1 from NACC inhibition. Reduced rat liver TrxR1 (0.34 unit/mL) was incubated with NACC (0.1 mM) in the presence or absence of DTNB (0.1, 0.2 and 0.4 mM) in PE buffer (100 mM potassium phosphate, pH 7.0, 2 mM EDTA) at room temperature for 15 min. An aliquot was withdrawn and tested for the remaining TrxR1 activity as described for the TrxR assay. The results are presented as the means ± S.D. of three independent experiments.
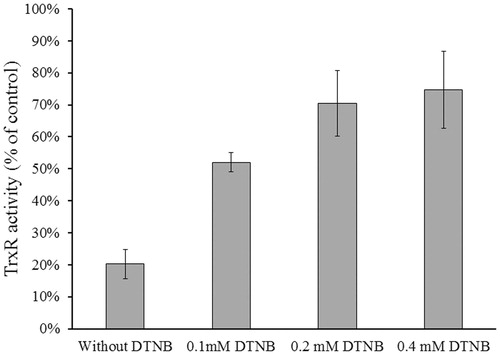
Figure 6. Determination of NADPH-dependent TrxR1 inhibition. Rat liver TrxR1 (0.34 unit/mL) was incubated with NACC (0.1 mM) in the presence or absence of NADPH (0.1 mM) in PE buffer at room temperature for 15 min. An aliquot was withdrawn and tested for the remaining TrxR1 activity as described for the TrxR assay. The results are presented as the means ± S.D. of three independent experiments.
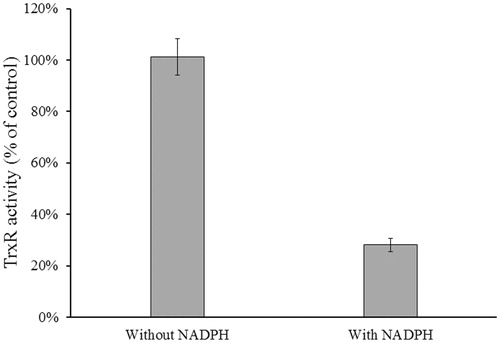
Figure 7. Mass spectrum of trypsinized NACC-treated TrxR1. (A) Mass spectrum of mono-p-chlorophenyl carbamoylated peptide –SGGDILQSGCUG containing active site of the enzyme. (B) Fragmentation of precursor ion m/z 649.19475 eluting at 33.5 min from trypsin-digested NACC-treated TrxR1. A series of fragment ions were observed including the ion m/z 379.99 which is the p-chlorophenyl carbamoylated y2 ion. (C) The extended tandem mass spectrum of (B) shows the chlorine element on the ion m/z 379.99 through isotope peak at m/z 381.99 confirming the p-chlorophenyl carbamoyl adduct.
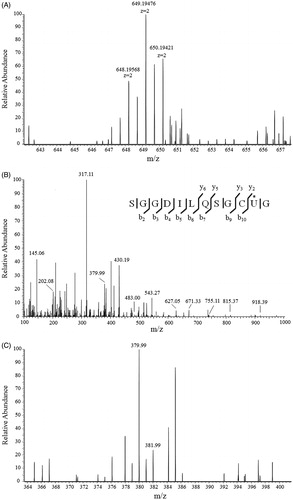
Figure 8. Proposed mechanism of TrxR1 inhibition by NACC. The tandem mass analysis of the NACC-inhibited TrxR1 revealed the selenothiols of Sec498 at the active site reacted with NACC to form mono-p-chlorophenyl carbamoylated enzymes, resulting in irreversible inhibition.
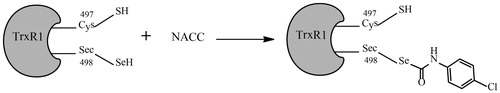
Table 1. Observed fragment ions of peptide –SGGDILQSGCU*G in the tandem mass spectrum of trypsin-digested NACC-treated TrxR1.
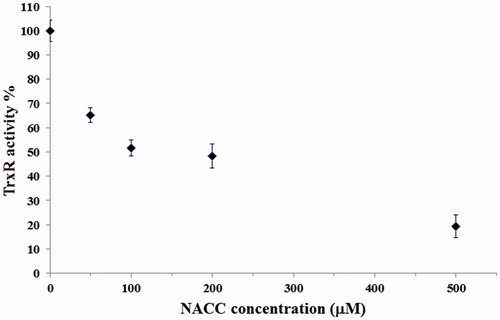
Figure 9. Inhibition of intracellular TrxR by NACC in UACC-62 cells. UACC-62 cells were incubated in the presence of 50, 100, 200 and 500 μM NACC for 5 h, and the enzyme activity was determined and presented as the percentage of the control. The results are shown as the means ± S.D. of three independent experiments.