Figures & data
Figure 1. Chemical structures of some selective cyclooxygenase-2 (COX-2) inhibitor drugs (1–3) and some traditional non-selective NSAIDs (4–6).
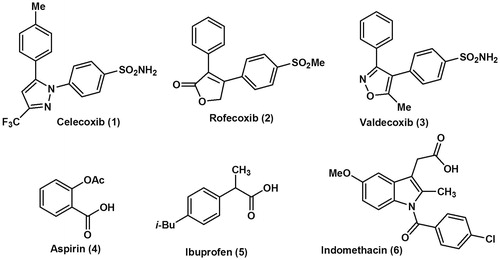
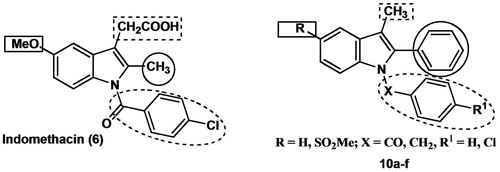
Figure 2. Chemical structures of the traditional NSAID indomethacin (6) and the designed indomethacin analogs 10a–f.
Scheme 1. Reagents and conditions: (a) glacial acetic acid, reflux, 10h; (b) benzoyl chloride, 4-chlorobenzoyl chloride or benzyl chloride, NaH, DMF, RT, overnight.
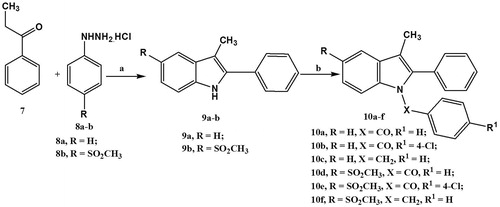
Table 1. In vitro COX-1 and COX-2 inhibition of compounds 10a–f, and reference drug (indomethacin).
Table 2. Anti-inflammatory activities of compounds 10a–f, and reference drug (indomethacin) in carrageenan-induced rat paw edema assay.
Figure 3. Analgesic effect of test compounds 10a–f compared to indomethacin using acetic acid-induced writhing in mice. Data represent the mean value ± SD of four mice per group. Statistical comparisons between basal and post-drug values were analyzed for statistical significance using the one-way ANOVA followed by Dunnett’s test and denoted by *p < 0.05, #p < 0.01.
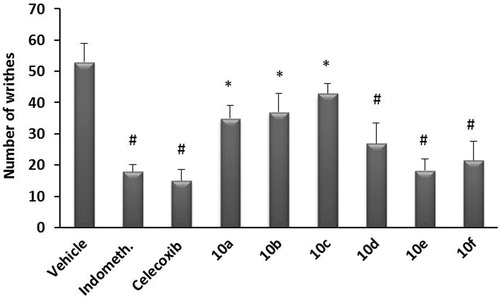
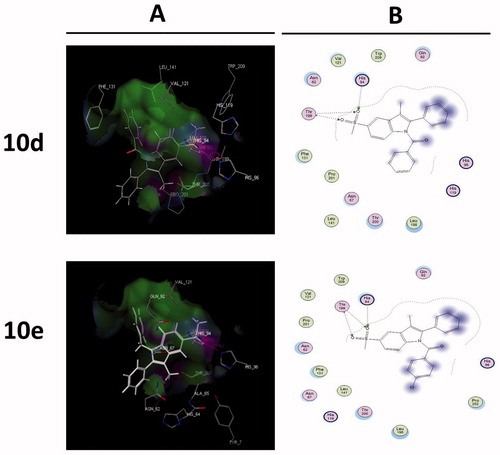
Figure 4. Binding of the most active compounds 10d and e inside COX-2 active site. (A) The proposed binding mode inside the active site of COX-2 resulting from docking, the most important amino acids are shown together with their respective numbers. (B) 2D interaction.