Figures & data
Figure 1. Chemical structures of resveratrol, NSC 14778 and aspirin. The hybrid resveratrol-salicylate derivatives possess the combined chemical features of these three different types of agents; the methylene bridge in NCS 14778 is replaced by an ethylene linkage between a phenol on one side, and the salicylate on the other.
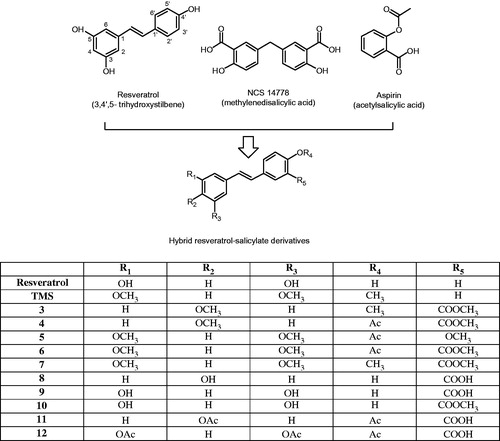
Figure 2. Chemical structures of representative examples of nucleoside and non-nucleoside DNMT inhibitors.
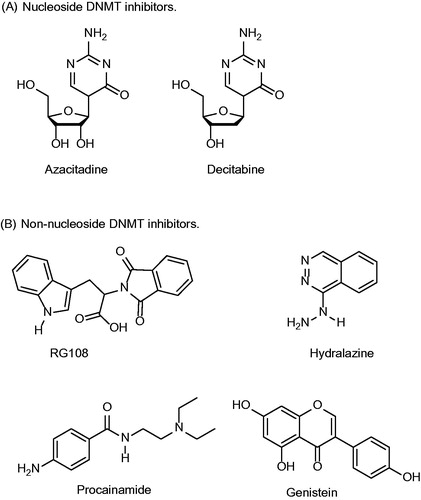
Table 1. Concentration (µM) of test compounds required to inhibit by 50% the enzymatic activity of DNMT1, DNMT3A, and DNMT3B.
Figure 3. Comparison of the binding modes calculated for compounds 9 (blue), 10 (purple), and resveratrol (orange) as predicted by AutoDock 4.2, in the presence and in the absence of the co-factor SAH (yellow) in the active site of DNMT1, DNMT3A and DNMT3B enzymes.
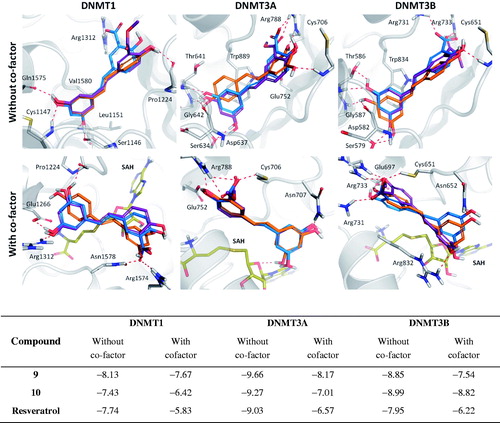
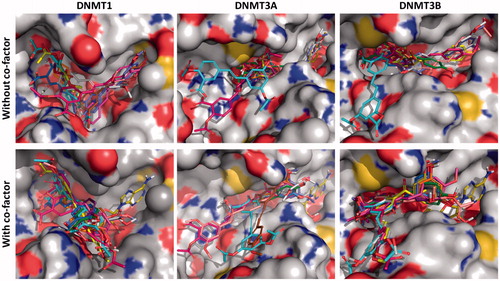
Figure 4. Comparison of the binding modes of compounds 3 (magenta), 4 (yellow), 5 (pink), 6 (gray), 7 (violet), 8 (green), 9 (blue), 10 (purple), 11 (pink), 12 (cyan), TMS (brown) and resveratrol (orange) predicted by AutoDock 4.2 in the presence and absence of co-factor SAH of DNMT1, DNMT3A and DNMT3B.