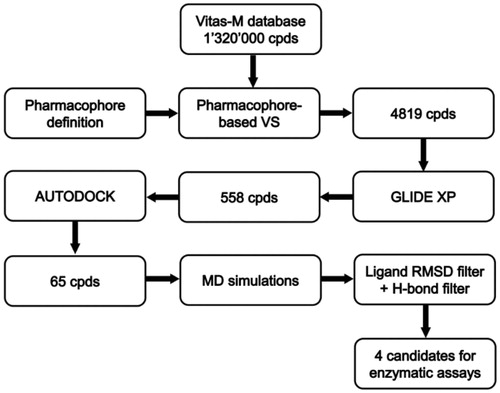
Figures & data
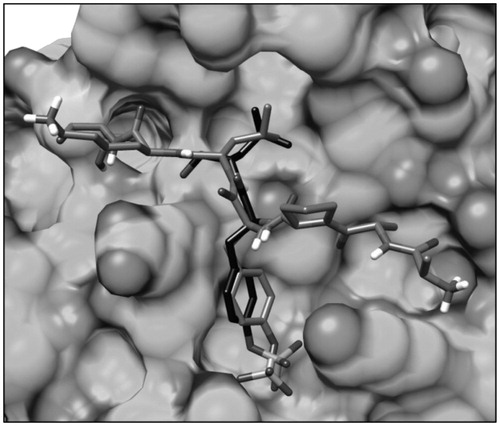
Figure 2. Structure of the phopshopeptide AAPpYLKT (stick), bound to the STAT3 SH2 domain (shown as protein surface) obtained from the structure of the STAT3β homodimer co-crystallized with a DNA fragment. The two pockets interacting with the residues pTyr705 and Leu706 of the phosphopeptide are highlighted.
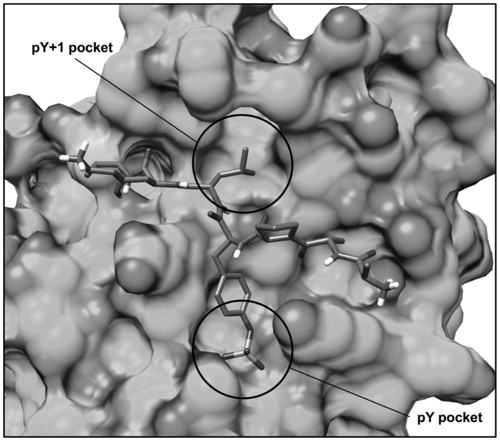
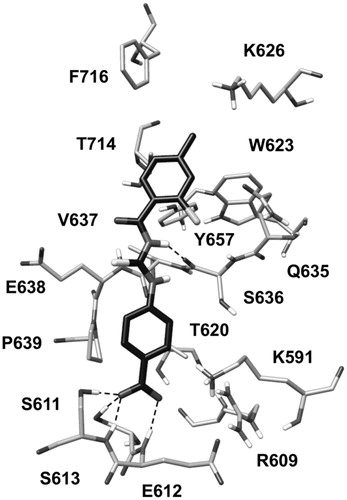
Figure 3. (A) Structure of the phosphopeptide AAPpYLKT (dark grey) bound to the STAT3 SH2 domain; the residues of the SH2 domain interacting with the pTyr705-Leu706 portion of the peptide and delimiting the pY pocket and the pY+1 pocket are shown (light grey). The main H-bonds detected in the complex are highlighted as black dashed lines. (B) Receptor-based pharmacophore model used for the VS study; the reference peptide (stick) together with the negative (N27), aromatic (R30), H-bond donor (D17) and hydrophobic (H24) features are shown; the excluded volume spheres are shown in light grey.
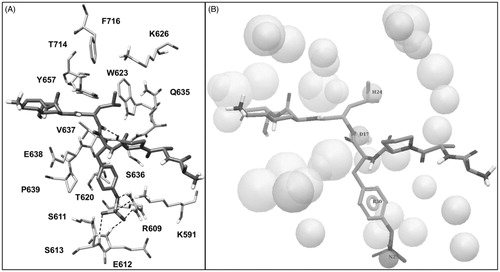
Figure 4. Structure of the pTyr705-Leu706 dipeptide (black stick) docked into the STAT3 SH2 domain (shown as protein surface) superimposed to the bound phosphopeptide AAPpYLKT (dark grey stick).