Figures & data
Figure 1 Binding of monoclonal antibodies to epitopes on SP-D. 245–1 binds to the neck area of SP-D, which may become less accessible when SP-D is polymerised. 246–4 binds to the lateral side of the CRD and blocks the binding of gp340 to SP-D. 246–2 binds to the lectin site and reduces the binding of viruses to SP-D.
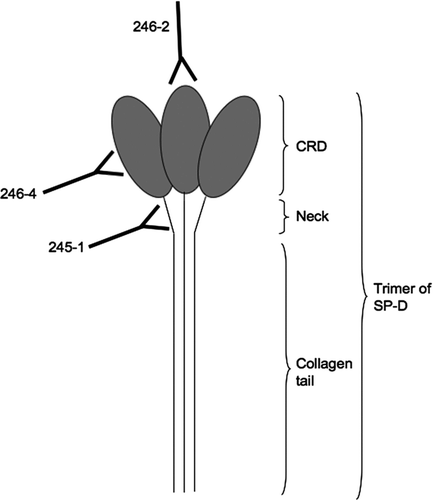
Figure 2 Cleavage of SP-D by neutrophil elastase. (A) 2 μg SP-D were cleaved at 37°C for 30 minutes with different dilutions (d) of neutrophil elastase (NE). This was then scaled up to prepare the antigen to immunise the mice (B). The proteins were separated on 12% w/v acrylamide SDS-PAGE and then visualised by silver staining. cSP-D: cleaved SP-D.
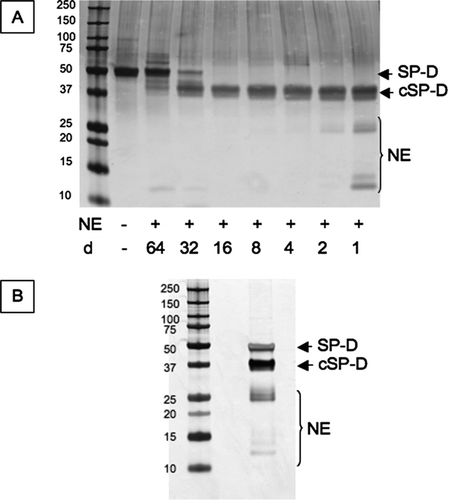
Figure 3 Screening of immunisation bleeds. The pre-immunisation bleed (day 0), first bleed (day 21) and terminal bleed (day 35) of each mouse ((A) –mouse 1; (B) –mouse 2) were used in antigen-mediated ELISA against SP-D, neutrophil elastase cleaved SP-D (cSP-D) and neutrophil elastase (NE). Values showed are maximum OD450 obtained.
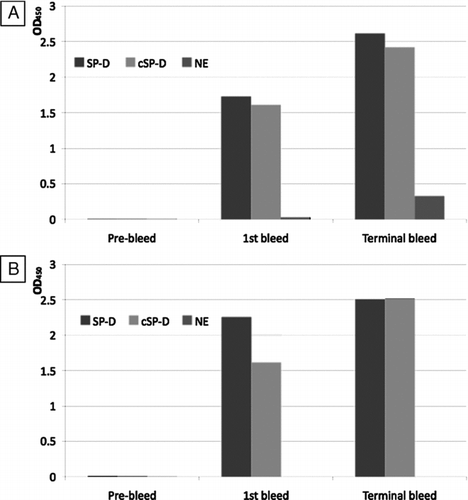
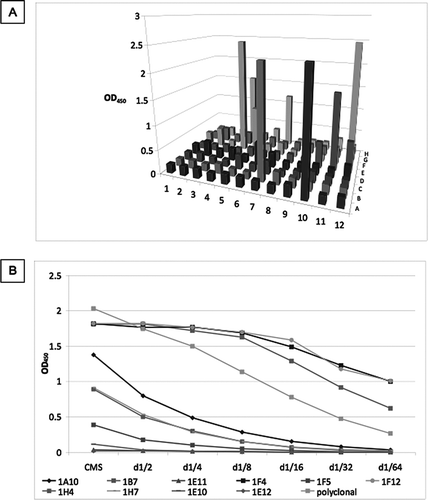
Figure 4 Screening of culture media supernatant (CMS) by antigen-mediated ELISA. (A) Fused cells were plated on a 96-well plate. CMS from all wells was screened against a mix of 1μg/ml of full length SPD and 1 μg/ml neutrophil elastase cleaved SP-D. Clones were named according to their position on the plate. (B) Positive wells were expanded in 12-well plates and rescreened against a mix of 1μg/ml of full length SPD and 1 μg/ml cleaved SP-D. Clones 1E11, 1F5, 1E10 and 1E12 were lost. After further expansion clones 1A10, 1H4, 1H7 were also lost. The remaining clones were screened against (C) full length SP-D and (D) cleaved SP-D. A polyclonal anti-SP-D antibody was used as a positive control (diluted 1/1000). d1/x: dilution of CMS. None of the antibodies detected neutrophil elastase.
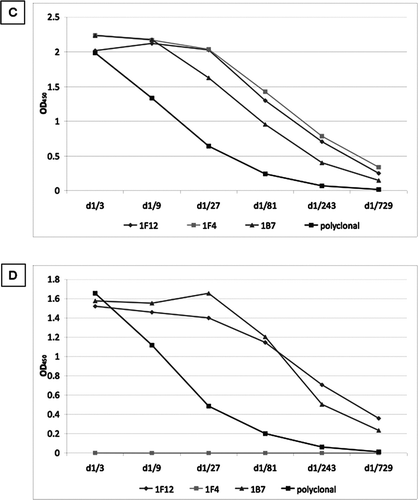
Figure 5 Characterisation of novel and commercially available antibodies. Full length SP-D and neutrophil elastase cleaved SP-D (cSP-D) were separated by a 10% w/v SDS-PAGE with or without 200mM DTT and (A) stained with Simply Blue SafeStain, or blotted with (B) polyclonal anti-SP-D antibody, (C) 1F4, (D) 246–4, (E) 246–2 or (F) 245–1 monoclonal antibodies.
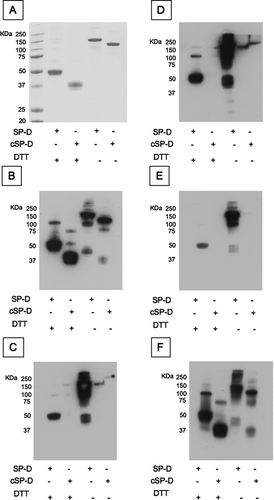
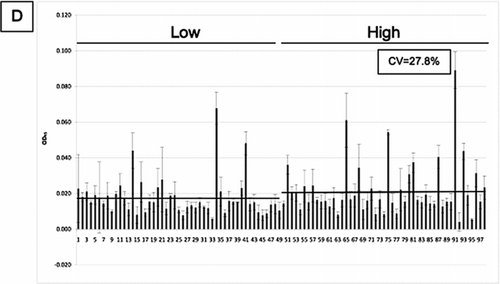
Figure 6 SP-D levels in 98 samples from the ECLIPSE cohort. SP-D levels were measured in serum samples by sandwich ELISA with 4 different antibodies ((A) 246–4, (B) 1F4, (C) 246–2 and (D) 245–1). CV: inter-assay coefficient of variation. Inserted graph: Representative standard curve. Low/high: Samples were chosen for their low/high SP-D levels according to previous measurements. Bars represent the average values. The mean values for the high and low samples were 83.431 (±37.1) and 46.899 (±14.16) ng/ml for monoclonal antibody 246–4 (A), 128.602 (±56.21) and 82.172 (±43.37) ng/ml for monoclonal antibody 1F4 (B), 133.858 (±62.2) and 102.348 (±78.13) ng/ml for monoclonal antibody 246–2 (C) and 0.021 (±0.0152) and 0.018 (±0.0109) units for monoclonal antibody 245–1 (D). Monoclonal antibody 245–1 did not recognise purified full length SP-D in ELISA and thus, no standard curve was constructed. Eight serum samples were added to each ELISA plate to control for intra-plate variation.
Table 1 Spearman correlation for the monoclonal antibodies 1F4, 245–1, 246–2 and 246–4 and the anti-SNO antibody with the components of the COPD phenotype
Figure 7 Statistical comparison of the values obtained with 5 different antibodies by bagplot. *: Bivariate median, dark grey: bag containing 50% of the observations with greatest bivariate depth, light grey: loop being the bag expanded 3 times, dot: Outlier being values outside the loop. (A) 1F4 vs 245–1, (B) 1F4 vs 246–4, (C) 1F4 vs 246–2, (D) 246–2 vs 246–4, (E) 245–1 vs 246–4, (F) 245–1 vs 246–2.
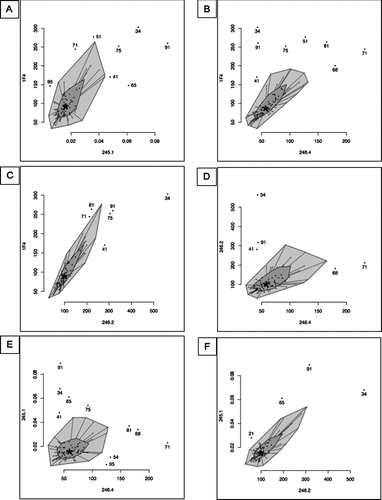
Figure 8 Western blot analysis of the outliers and controls (Low: lowest quartile, High: highest quartile of SP-D levels). Serum SP-D was purified by binding to maltose-agarose beads and analysed by western blot with (A) a polyclonal anti-SP-D antibody, (B) 1F4, (C) 246–4, (D) 246–2 or (E) 245–1 antibody.
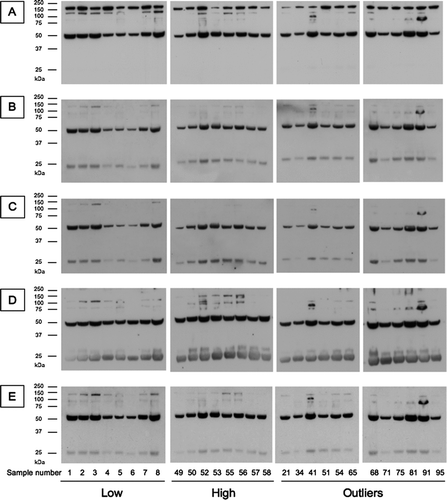
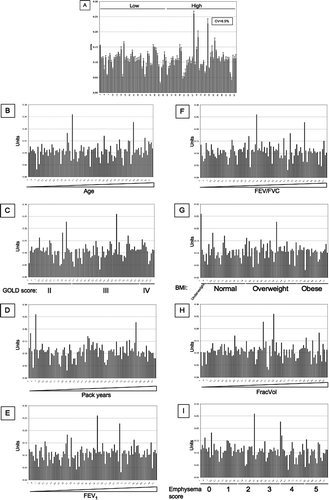
Figure 9 SNO-SP-D levels in 98 samples from the ECLIPSE cohort. (A) SNO-SP-D levels were measured in serum samples by sandwich ELISA. CV: Inter-assay coefficient of variation. Low/high: Samples chosen for their low/high SP-D levels according to previous measurements. Values were then arranged by increasing age (B), GOLD stages (C), increasing pack years (D), increasing FEV1 (E), increasing FEV1/FVC (F), BMI (G), increasing FracVol (H) and emphysema scores (I).
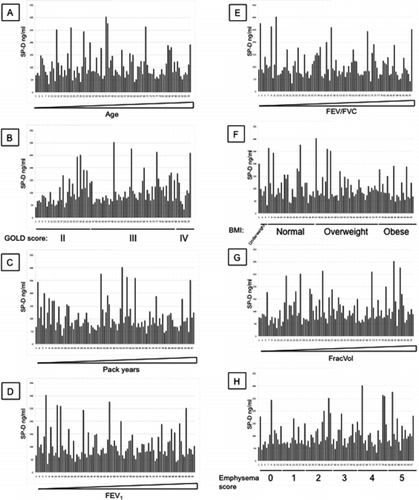
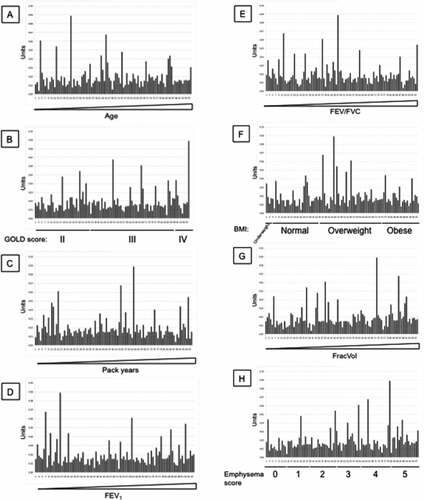
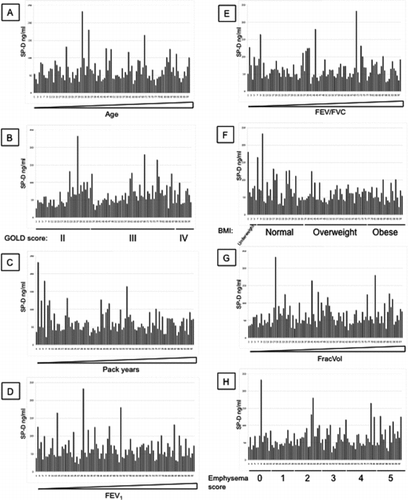
Additional File 1 SP-D levels measured with antibody 246–4 were arranged by increasing age (A), GOLD stages (B), increasing pack years (C), increasing FEV1 (D), increasing FEV1/FVC (E), BMI (F), increasing FracVol (G) and emphysema scores (H).
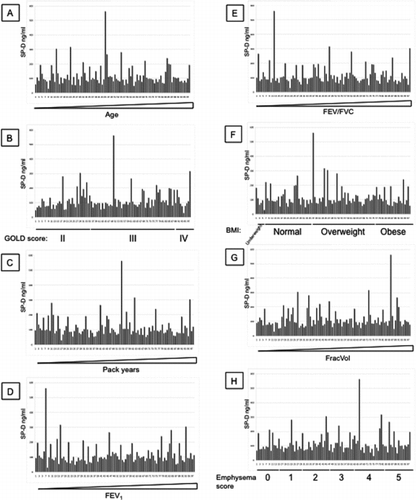
Additional File 2 SP-D levels measured with antibody 1F4 were arranged by increasing age (A), GOLD stages (B), increasing pack years (C), increasing FEV1 (D), increasing FEV1/FVC (E), BMI (F), increasing FracVol (G) and emphysema scores (H).