Figures & data
Table 1. Patient flow-through studies
Table 2. Demographics and baseline characteristics
Figure 1. TDI total score at weeks 4 and 12. Data are least squares means ± standard errors. **p < 0.01, ***p ≤ 0.001 versus placebo. TDI = transition dyspnea index.
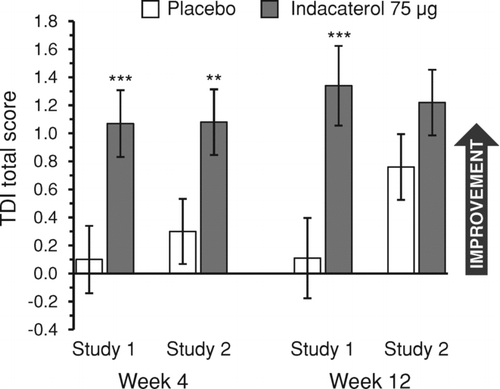
Figure 2. Change from baseline in SGRQ total score at weeks 4 and 12. Data are unadjusted (raw) means (week 4 data are without imputation). Broken line indicates level of clinically relevant improvement. SGRQ = St George's Respiratory Questionnaire.
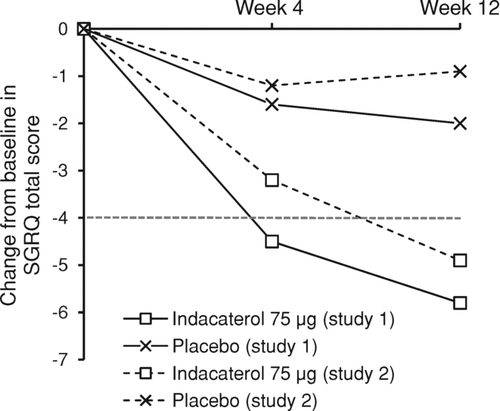
Figure 3. Percentages of patients with a clinically important improvement in TDI total score and SGRQ total score after 12 weeks of treatment, with associated odds ratios (OR) and p-values for the indacaterol–placebo differences (*p < 0.05, **p < 0.01 versus placebo). TDI = transition dyspnea index; SGRQ = St George's Respiratory Questionnaire.
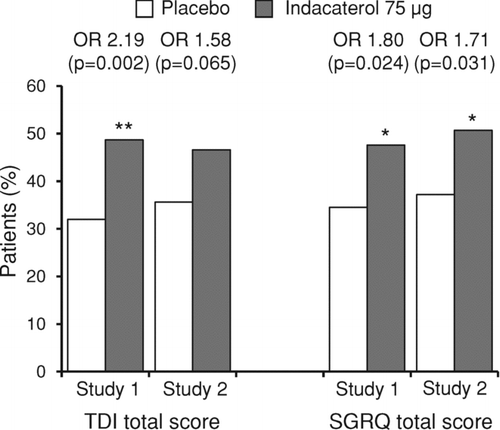
Table 3. TDI and SGRQ results in patient subgroups according to COPD severitya
Table 4. Number and rate of exacerbations of COPD