Figures & data
Figure 2. Flow chart for patient entry. NETT: National Emphysema Treatment Trial. N: Number of patients.
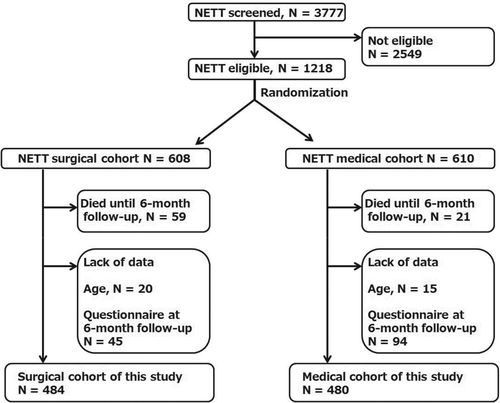
Table 1. Baseline characteristics and longitudinal changes in questionnaire scores
Figure 3. Distribution chart for ∆SGRQ and ∆SOBQ. The gray bars indicate the cutoff values for ∆SGRQ ± 1 (for anchor average method). The oblique line indicates the linear regression (for anchor linear regression method).
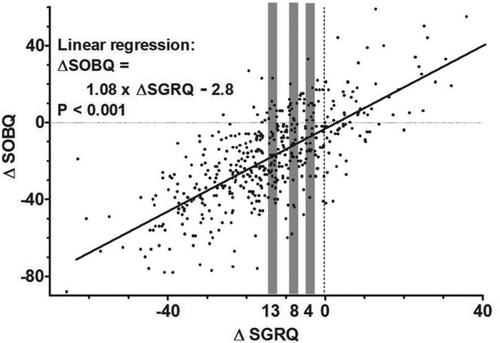
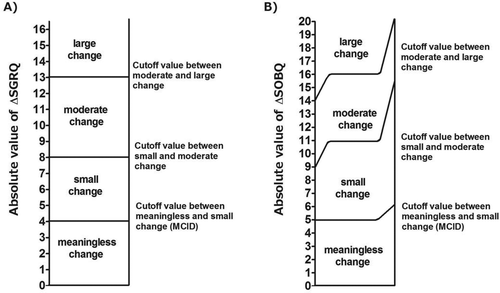
Table 2. Summary of the estimated cutoff values of meaningless, small, moderate, and large changes in Shortness of Breath Questionnaire (SOBQ)
Table 3. A comparison of the numbers of patients in each category when grouped according to other parameters