Figures & data
Table 1. Demographics and baseline characteristics of the study population (safety population)
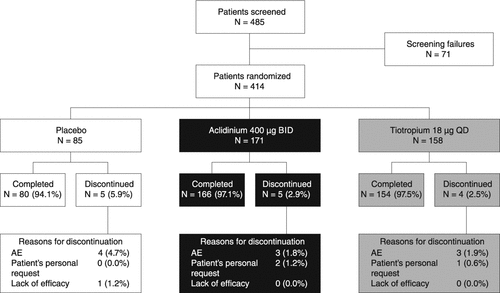
Figure 2. Change from baseline in FEV1 AUC0–24, FEV1 AUC12–24, and FEV1 AUC0–12 compared with placebo (A) on day 1 and (B) at week 6 (ITT population). Data reported as LS mean (95% CI) differences from placebo (ANCOVA). ‡p < 0.001; §p < 0.0001 for aclidinium or tiotropium versus placebo. ¶p < 0.05; #p < 0.01 for aclidinium versus tiotropium. ANCOVA, analysis of covariance; AUC, area under the curve; AUC0–24, AUC over the 24-hour period post-morning treatment; AUC12–24, AUC over the nighttime period; AUC0–12, AUC for the 12-hour period post-morning treatment; BID, twice daily; CI, confidence interval; FEV1, forced expiratory volume in 1 second; ITT, intent-to-treat; LS, least squares; QD, once daily.
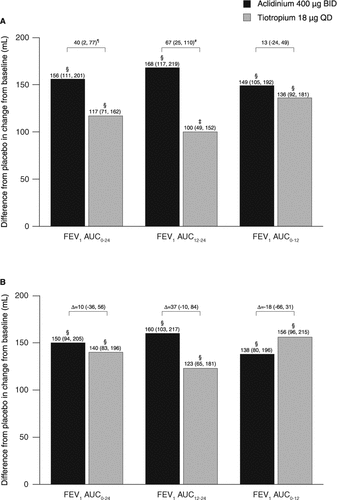
Figure 3. Change from baseline in FEV1 over 24 hours (A) on day 1 and (B) at week 6. Data reported as LS mean change from baseline (ANCOVA). p < 0.01 for aclidinium and tiotropium versus placebo at each time point. ¶p < 0.05; #p < 0.01; ∥p < 0.001; ¶¶p < 0.0001 for aclidinium versus tiotropium. ANCOVA, analysis of covariance; BID, twice daily; FEV1, forced expiratory volume in 1 second; LS, least squares; QD, once daily.
Table 2. Change from baseline (difference from placebo) in additional lung-function variables (ITT population)
Figure 4. Change from baseline in E-RS total and domain scores over 6 weeks. Data reported as LS mean (95% CI) difference from placebo (ANCOVA). *p < 0.05; †p < 0.01; §p < 0.0001 for aclidinium or tiotropium versus placebo. ANCOVA, analysis of covariance; BID, twice daily; CI, confidence interval; E-RS, EXAcerbations of Chronic pulmonary disease Tool (EXACT)-Respiratory Symptoms; LS, least squares; QD, once daily.
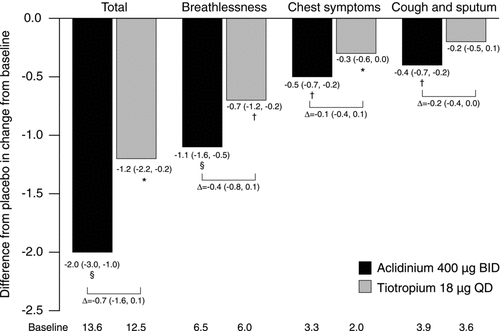
Figure 5. Difference from placebo in change from baseline in (A) severity of early-morning symptoms, (B) severity of nighttime symptoms and number of nocturnal awakenings due to COPD symptoms, and (C) limitation of activity caused by COPD symptoms (COPD additional symptoms questionnaire) over 6 weeks (ITT population). Data reported as LS mean differences from placebo (ANCOVA). *p < 0.05; †p < 0.01; ‡p < 0.001 for aclidinium or tiotropium versus placebo. ¶p < 0.05 for aclidinium versus tiotropium. Severity of overall early-morning and nighttime symptoms rated on a 5-point scale from 1 = ‘did not experience any symptoms’ to 5 = ‘very severe’; individual morning symptoms rated on a 5-point scale from 0 = ‘no symptoms’ to 4 = ‘very severe’; limitation of activity rated on a 5-point scale from 1 = ‘not at all’ to 5 = ‘a very good deal’. ANCOVA, analysis of covariance; BID, twice daily; COPD, chronic obstructive pulmonary disease; ITT, intent-to-treat; LS, least squares; QD, once daily.
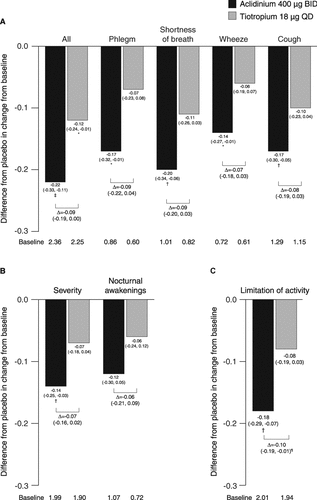
Table 3. Patient preference for each inhaler based on specific inhaler attributes at week 6, n (%)