Figures & data
Table 1. Baseline characteristics of 610 participants randomized to medical treatment in the National Emphysema Treatment Trial, by chronic bronchitis status and by severe chronic bronchitis status
Figure 1. Mortality in A) the chronic bronchitis versus no chronic bronchitis groups and B) the severe chronic bronchitis versus no severe chronic bronchitis groups.
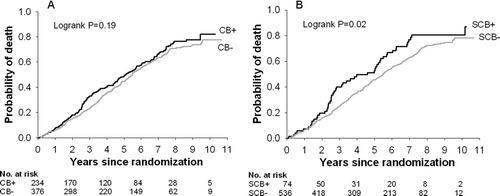
Figure 2. Time to first hospitalization in A) the chronic bronchitis versus no chronic bronchitis groups and B) the severe chronic bronchitis versus no severe chronic bronchitis groups.
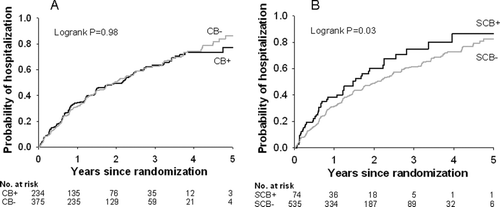
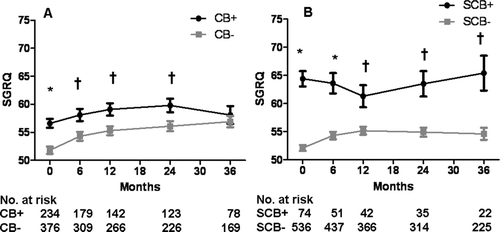
Figure 3. A) Time to death and B) time to hospitalization in those reporting chest trouble most days per week and not reporting cough and phlegm most or several days per week (CT+) versus those with little or no chest trouble and not reporting cough and phlegm most or several days per week (CT−). Those reporting cough and phlegm most or several days per week are excluded from both the CT+ and CT− groups.
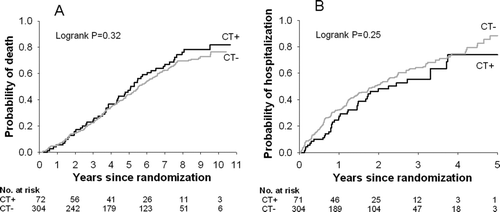
Table 2. Time from randomization to death and time from randomization to first hospitalization, by chronic bronchitis status and severe chronic bronchitis status for 610 patients randomized to medical treatment in the National Emphysema Treatment Trial
Table 3. Multivariate models of time to death and first hospitalization
Figure 4. Lung function at baseline and during follow-up for the CB+ and CB− groups. A) forced vital capacity, B) forced expiratory volume in 1 second, C) total lung capacity, and D) residual volume. Data presented as mean ± SE. *p < 0.05,**p < 0.01,†p < 0.005.
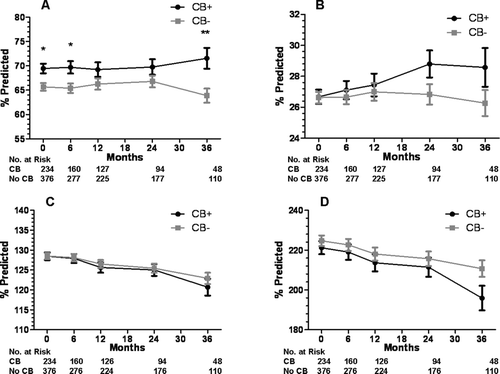
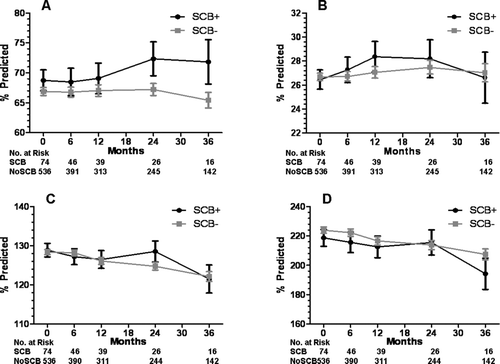
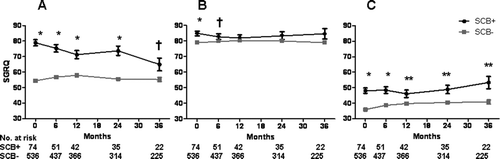
Figure 5. Lung function at baseline and during follow-up for the SCB+ and SCB− groups. A) forced vital capacity, B) forced expiratory volume in 1 second, C) total lung capacity, and D) residual volume. Data presented as mean ± SE.