Figures & data
Figure 1. Effect of FN on MMP-9, TIMP-1, and α5 integrin in PC-3 cells: A. Cell-adhesion assay was done with different concentrations (2.5, 5, and 10 μg/ml) of FN and percentages of adhesion were plotted. B. PC-3 cells were grown in SFCM in absence (Lane 1) and in presence of 10 μg/ml of FN for 2 h (Lane 2), for 4 h (Lane 3), and 10 μg/ml of LN for 4 h (Lane 4). Culture supernatant of HT-1080 cells grown in SFCM for 24 h was used as MMP-9 (92 kDa)/MMP-2 (72 kDa) marker (Lane -M). The culture supernatants in all the cases were subjected to gelatin zymography. C. PC-3 cells were grown in serum-free culture medium (SFCM) in absence (0) and in presence of 10 μg/ml FN for 4 h. 2 steps RT-PCR was done with equal amounts of total RNA, using MMP-9 and TIMP-1 specific primers for PCR. GAPDH primers were used to confirm equal loading. D. PC-3 cells were treated without (Lane 1) or with 1 μg/ml anti- α3 (Lane 2), anti-α4 (Lane 3), and anti-α5 (Lane 4) integrin antibodies for 1 h and then all the sets were grown in presence of 10 μg/ml FN for 4 h. Gelatin zymography of the culture supernatants were performed. E. Following FN treatment (10 μg/ml for 4 h), PC-3 cells were collected, extracted, and equal amounts of protein (100 μg) was subjected to western blot analysis with anti-α5 antibody (1 μg/2 ml dilution) and respective HRP-coupled secondary antibody (1 μg/200 ml dilution) for ECL method. β-tubulin was used as internal control.
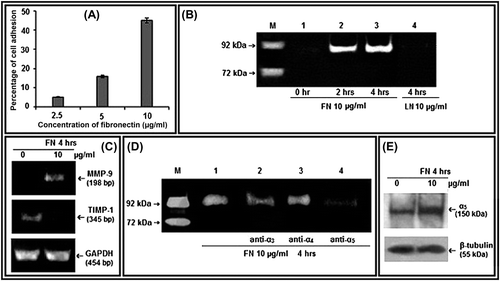
Figure 2. Effect of FN on FAK and PI3K, and their involvement in FN-induced MMP-9 activity: A. PC-3 cells, untreated (Lane 1) or treated with PI-3K inhibitor LY 294002 (Lane 2); ERK inhibitor PD 98059 (Lane 3) and p38 inhibitor SB 203580 (Lane 4), were grown in presence of FN and gelatin zymography of the culture supernatants were performed. B. PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of proteins (100 μg) of cell extracts were subjected to western blot analysis with anti-FAK, anti-p-FAK, anti-PI3K, anti-p-PI3K antibodies. β-tubulin was used as internal control. C. PC-3 cells were grown on coverslips in absence (Control) and presence of FN (10 μg/ml) for 1, 2, 4 h. Immunocytochemistry was preformed with anti-FAK (1 μg/ml dilution) primary and respective FITC-labeled secondary antibody (1 μg/ml dilution). White arrows and yellow arrows show localization of FAK at focal points and nucleus, respectively. D. PC-3 cells were transfected with control and FAK siRNA (60 nM each) followed by FN treatment and then culture supernatant was subjected to zymography (d) and cell extracts were subjected to western blot analysis of FAK (a), p-PI3K (b). β-tubulin (c) was used as internal control for western blot. Lane 1 is control lane without FN or siRNA treatment, Lane 2 denotes treatment of FN (10 μg/ml for 4 h) without siRNA treatment, Lane 3 is with FN and FAK siRNA treatment, Lane 4 is with FN and control siRNA treatment.
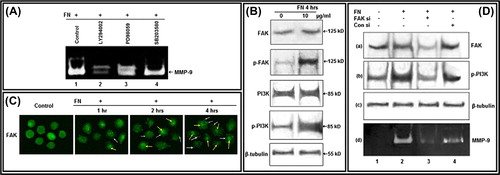
Figure 3. Effect of FN on Akt, NF-κB: A. PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-Akt, anti-p-Akt (Thr-308), anti-p-Akt (Ser-473) antibodies. β-tubulin was used as internal control. B. Nuclear and cytoplasmic extracts of control (0) and FN-treated (10 μg/ml) cells were subjected to western blot analysis with anti-NF-κB antibody. TBP was used as internal control for nuclear proteins. C. PC-3 cells, untreated (Control) or treated with NF-κB inhibitor BAY-11-7085 (5 μM for 24 h), were allowed to grow on FN (10 μg/ml for 4 h in SFCM). The culture supernatants were collected and gelatin zymography was performed.
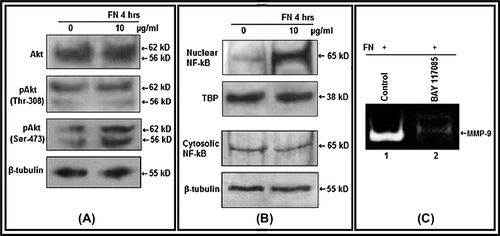
Figure 4. Effect of FN on PC-3 cell migration, invasion and cell surface expression of CD-44: A. Wound-healing assay of PC-3 cells grown without (-FN) or with FN (+ FN). B. Transwell assay. C. Immunocytochemistry was preformed with anti-CD-44 (1 μg/ml dilution) primary and respective biotin-labeled secondary antibody (1 μg/ml dilution).
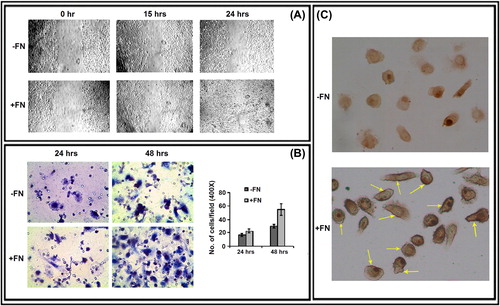
Figure 5. Effect of FN on E-cadherin, VEGF and ILK: PC-3 cells were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-E-cadherin, anti-VEGF, anti-ILK, anti-p-ILK antibodies. β-tubulin was used as internal control.
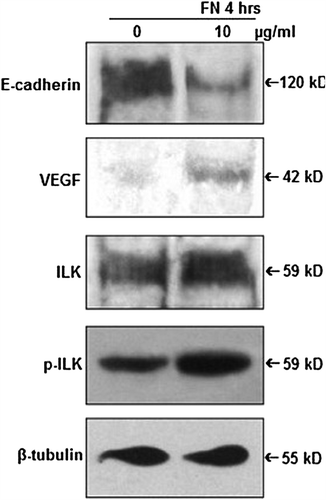
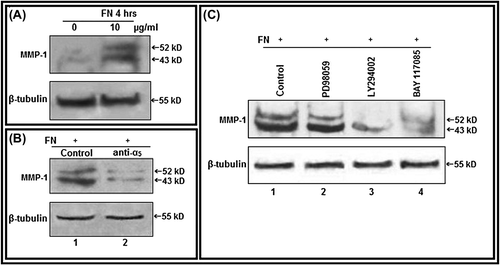
Figure 6. Culture of PC-3 cells on FN-coated surface modulate MMP-1: A. PC-3 cells (300 000 cells/ml) were grown in SFCM in absence (0) and in presence of 10 μg/ml FN for 4 h. Equal amounts of protein (100 μg) of cell extracts were subjected to western blot analysis with anti-MMP-1 antibody. B. PC-3 cells, untreated or treated with anti-α5 integrin antibody (1 μg/ml for 1 h), were allowed to grow in presence of FN (10 μg/ml for 4 h). Cell extracts (100 μg) were subjected to western blot analysis with anti-MMP-1 antibody. C. PC-3 cells, untreated (Lane 1) or treated with ERK inhibitor PD98059 (Lane 2), PI-3K inhibitor LY294002 (Lane 3) (50 μM each in SFCM for 1 h) and NF-κB inhibitor (Lane 4) BAY-11-7085 (5 μM for 24 h), were allowed to grow in presence of FN (10 μg/ml for 4 h). Cell extracts (100 μg) were subjected to western blot analysis with anti-MMP-1 antibody. β-tubulin was used as internal control.