Figures & data
Figure 1. Phagocytosis of irradiated, apoptotic tumor cells (ITC) by macrophages or dendritic cells (DC) in the presence or absence of AnxA5. Irradiated RMA tumor cells (ITC) were co-incubated at different ratios with thioglycollate-elicited Mϕ (A) or DC (B) from C57BL/6 mice for 2 h. The phagocytosis (analyzed by flow cytometry) at 37°C of ITC by Mϕ or DC in the presence of medium only was set to 100. The percentage of inhibition of phagocytosis in the presence of AnxA5 (100 μg/mL) or BSA (100 μg/mL) is displayed. Results are representative of five independent experiments (each performed in quadruplicate) are expressed as percentage of inhibition (±SD). AnxA5, in contrast to the control peptide BSA, significantly inhibited uptake of ITC by macrophages, but not by DC, at all ratios tested **P < 0.01 vs BSA.
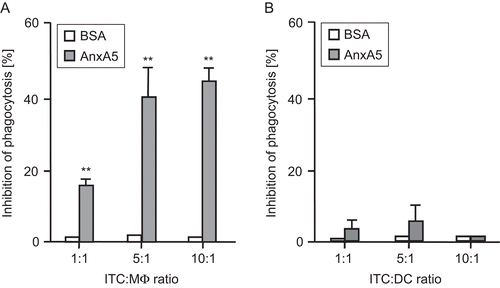
Figure 2. AnxA5 modulates the cytokine secretion profile of macrophages after contact with irradiated, apoptotic tumor cells (ITC). Irradiated RMA tumor cells (ITC) were co-incubated, in the presence or absence of AnxA5, at a ratio of 5:1 with thioglycollate-elicited peritoneal Mϕ from C57BL/6 mice for 24 hr at 37°C. The culture of Mϕ in medium (m) alone or in medium supplemented with AnxA5 (100 μg/mL) served as controls. The supernatants were retrieved and the release of TNFα, IL-1β, IL-10, and TGF-β determined using ELISA. The results from one representative experiment (each performed in quadruplicate) are displayed. *P < 0.05; **P < 0.01.
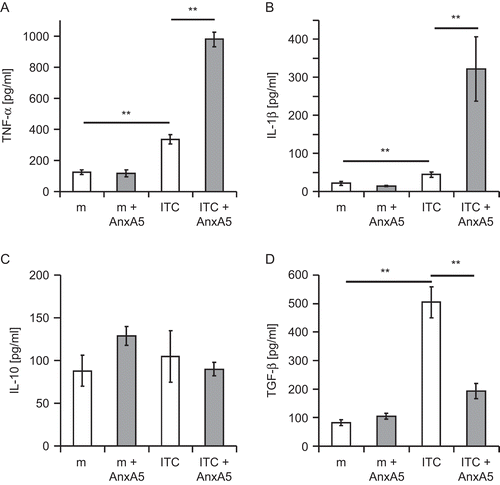
Figure 3. Influence of endogenous levels of AnxA5 on phagocytosis of necrotic tumor cells and on regression of an allogeneic tumor. Macrophages in the peritoneum of AnxA5 KO and WT mice had contact with necrotic WEHI 231 cells for 3.5 h. Following lavage of the peritoneum, the percentage of highly phagocytosing macrophages (Mϕ) which have taken up more than one necrotic cell was determined using flow cytometry. The results are obtained from lavages of four WT and six AnxA5 KO mice (A). The uptake by macrophages of AnxA5 KO mice of necrotic cells was significantly enhanced (P < 0.01) in comparison to the WT situation. The tumor diameter of the allogeneic CT26 tumor 2 wk after the injection in AnxA5 KO or WT mice is displayed in (B). Note: WT mice showed a significant faster regression of the tumor. One out of three independent experiments (each performed with at least five mice per group) are displayed. **P < 0.01.
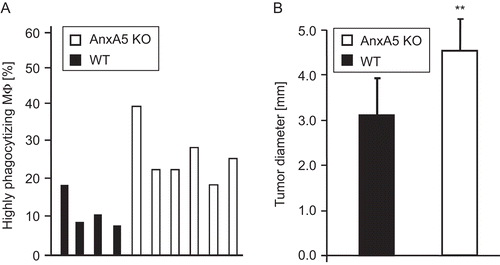
Table 1. Percentage of tumor free-mice after vaccination with irradiated tumor cells (ITC) only or with ITC plus AnnexinA5 (AnxA5).
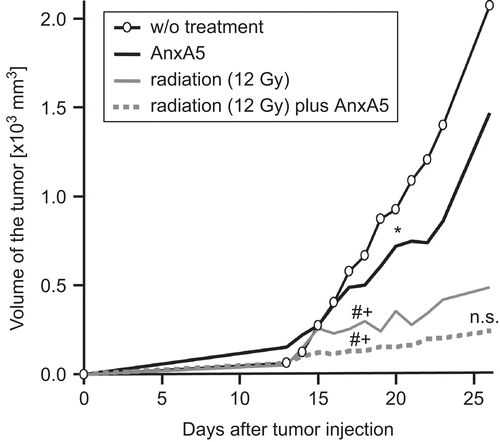
Figure 4. Growth of a syngeneic tumor is inhibited by ionizing irradiation and by treatment with AnxA5. CT26 tumor cells were subcutaneously injected (on Day 0) into Balb/c mice and tumor growth was monitored thereafter by measures of tumor volume. Irradiation was performed with a single dose of 12 Gy at Day 14, and AnxA5 (40 μg/100 mm3) was injected on Days 14–23. One representative experiment (n = 10) out of three is displayed. The median tumor volume values of tumors of ten different mice are shown. Indications for statistical significance are given for the first timepoint at which the significant difference became evident and remained thereafter, and are marked as follows: *: AnxA5 vs. w/o treatment; #: radiation or radiation plus AnxA5 vs. AnxA5; +: radiation or radiation plus AnxA5 vs. w/o treatment; n.s.: radiation vs. radiation plus AnxA5. *P < 0.05; #P < 0.01; +P < 0.01. n.s.: nonsignificant; w/o: without.