Figures & data
Figure 1. Interplay between microbiota and mucosal immunity in the gut. The figure shows a schematic view of the multiple relationships between commensal bacteria (cb), mucosal cells, and immune cells involved in the gut immune responses and homeostasis. Bacteria (b) on the mucosal surface are inhibited from invading the mucosa by both mechanical-anatomical barrier (mucus, tight intercellular junctions) and mucosal immunity activation. Immune cells involved in this network are mainly located in the lamina propria and within mucosal epithelial structures (intraepithelial lymphocytes, IEL). Innate immunity cells (e.g., macrophages, Mϕ; mast cells, MC; natural killer cells, NK) are involved (along with local dendritic cells [DC]) in recognition, killing, processing, and presentation of bacterial-/food-derived antigens to adaptive immunity T-cells (T) that are then primed and activated. These innate immunity cells, once having been activated following contact with the bacteria, generate pro-inflammatory molecules like TNFα, IL-1β, NO, and/or PGE2. If the antigens are processed passing through the lamina propria defense network, DC activation of T-helper cells in the mesenteric lymph nodes (MLN, cell maturation centers) will lead naïve (TH0) cells to develop into regulatory/tolerant (i.e., TH2, TH3, Treg) cells. The cytokines that these specific cell types produce (e.g., IL-4, IL-10, TGFβ) are essential in modulating the continuously activated mucosal immunity (“physiologic inflammation”) and for inducing IgA production by lamina propria-associated B-cells. These IgA are secreted in the mucosa (to protect it from the target microflora) and circulate systemically to induce/partake in general immunologic effects.
Part of the gut local immune response is an acute inflammatory response. This is elicited by direct contact of either bacteria or intestinal lumen antigens with immune cells “skipping” along the lamina propria barrier or via stimulation of these cells by bacterial products like lipopolysaccharides. In this case, the response is the maturation of TH1 cells, the release of TH1 cytokines (IL-2, IFNγ, TNFα), and the elicitation of cytotoxic activity. If these responses are not rigorously controlled and regularly terminated, damage to the mucosa can develop and the “physiological inflammation” can exceed the homeostatic limit and so become pathological. Activated (including tolerant) cells can pass into the lymphatics and blood vessels, entering the general circulation to impact upon systemic immune responses.
![Figure 1. Interplay between microbiota and mucosal immunity in the gut. The figure shows a schematic view of the multiple relationships between commensal bacteria (cb), mucosal cells, and immune cells involved in the gut immune responses and homeostasis. Bacteria (b) on the mucosal surface are inhibited from invading the mucosa by both mechanical-anatomical barrier (mucus, tight intercellular junctions) and mucosal immunity activation. Immune cells involved in this network are mainly located in the lamina propria and within mucosal epithelial structures (intraepithelial lymphocytes, IEL). Innate immunity cells (e.g., macrophages, Mϕ; mast cells, MC; natural killer cells, NK) are involved (along with local dendritic cells [DC]) in recognition, killing, processing, and presentation of bacterial-/food-derived antigens to adaptive immunity T-cells (T) that are then primed and activated. These innate immunity cells, once having been activated following contact with the bacteria, generate pro-inflammatory molecules like TNFα, IL-1β, NO, and/or PGE2. If the antigens are processed passing through the lamina propria defense network, DC activation of T-helper cells in the mesenteric lymph nodes (MLN, cell maturation centers) will lead naïve (TH0) cells to develop into regulatory/tolerant (i.e., TH2, TH3, Treg) cells. The cytokines that these specific cell types produce (e.g., IL-4, IL-10, TGFβ) are essential in modulating the continuously activated mucosal immunity (“physiologic inflammation”) and for inducing IgA production by lamina propria-associated B-cells. These IgA are secreted in the mucosa (to protect it from the target microflora) and circulate systemically to induce/partake in general immunologic effects.Part of the gut local immune response is an acute inflammatory response. This is elicited by direct contact of either bacteria or intestinal lumen antigens with immune cells “skipping” along the lamina propria barrier or via stimulation of these cells by bacterial products like lipopolysaccharides. In this case, the response is the maturation of TH1 cells, the release of TH1 cytokines (IL-2, IFNγ, TNFα), and the elicitation of cytotoxic activity. If these responses are not rigorously controlled and regularly terminated, damage to the mucosa can develop and the “physiological inflammation” can exceed the homeostatic limit and so become pathological. Activated (including tolerant) cells can pass into the lymphatics and blood vessels, entering the general circulation to impact upon systemic immune responses.](/cms/asset/fab57400-4cc3-453d-bb1c-2901241c2597/iimt_a_433612_f0001_b.gif)
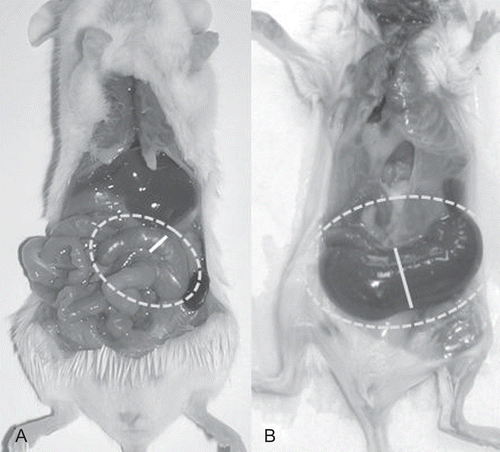
Figure 2. Conventional and germ-free (GF) reared mice. (A) Conventionally reared mouse (CV); (B) GF reared mouse. The cecum of both mice is framed by the interrupted line; the continuous line indicates the diameter of the cecum in its middle part. Differences in dimension are clearly evident. The GF animal has a very large and smooth cecum; its dark color is due to visibility of fecal content through a very transparent wall, and also due to a biliary acid metabolism that differs from that in conventional mice. In addition, the small bowel appears larger than in the CV animal control.