Figures & data
Figure 1. The Danger Hypothesis applied to IDRs. The drug or its reactive metabolite can form antigenic adducts with endogenous molecules that are presented to T-cells on major histocompatibility complexes of antigen-presenting cells (Signal 1). Additionally, the drug or reactive metabolite may induce cell stress or damage through processes such as redox cycling and oxidative damage. This may lead to the release of danger signals to up-regulate co-stimulatory molecules such as B7 and CD40 on antigen-presenting cells to activate T-cells (Signal 2). Signals 1 and 2 are required concurrently to initiate an adaptive immune response and certain drugs have the ability to induce both signals.
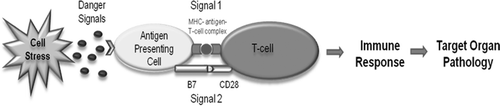
Figure 2. Metabolic scheme for the formation of reactive metabolites of aromatic amine drugs. Oxidation of aromatic amines to hydroxylamines can occur through enzymes such as CYP450s or myeloperoxidase (MPO); this is prevented by N-acetylation. Hydroxylamines are not very reactive but can auto-oxidize to electrophilic nitroso metabolites. The nitrosoamine can react with thiol-containing nucleophiles such as glutathione or endogenous proteins to form sulfinamide adducts. Additionally, the nitrosoamine can be reduced back to the parent drug, leading to redox cycling and oxidative stress.
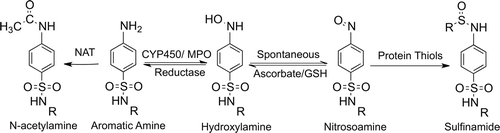
Table 1. Similar genes differentially regulated at least 1.4-fold change in the same direction in all aromatic amine drugs tested 12 h after treatment.
Figure 4. Eiih expression induced by aromatic amine drugs 12 h after treatment. Among the treatment groups (solid bars), AMG induced a significant increase in Eiih expression as compared to control (open bars). DDS also induced an increase in Eiih; however, no change was observed for SMX. Rats were given 150 mg SMX/kg, 20 mg DDS/kg, or 80 mg AMG/kg, and liver samples were taken for RT-PCR analysis. Eiih expression was normalized to β2-microglobulin (β2M) as a housekeeping gene and a calibrator control. RT-PCR was run in triplicate (n = 4; *** p < 0.001).
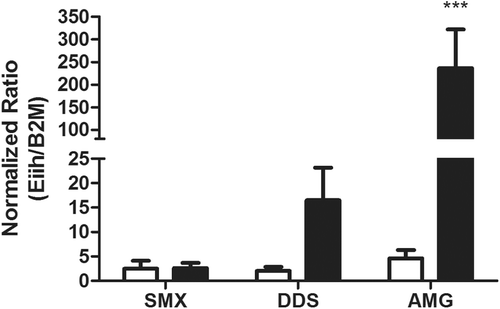
Figure 5. Time-course of hepatic Eiih expression induced by AMG. Eiih was expressed the greatest 8 and 12 h after AMG treatment (solid bars) as compared to control (open bars). Rats were treated with 80 mg AMG/kg, and liver samples were taken for RT-PCR analysis. Eiih expression was normalized to β2-microglobulin (β2M) as a housekeeping gene and a calibrator control. RT-PCR was run in triplicate (n = 2).
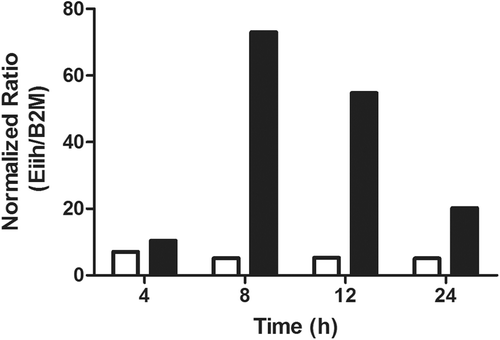
Figure 6. Hepatic gene expression of Eiih induced by several other drugs associated with IDRs. Clozapine (CLZ), an atypical anti-psychotic drug, induced an increase in Eiih 12 h after treatment of rats, and nevirapine (NVP), an anti-retroviral drug, increased Eiih expression at both 6 and 12 h after treatment of rats. Data expressed as fold-change between treated and controls (n = 4; * p < 0.05, ** p < 0.01).
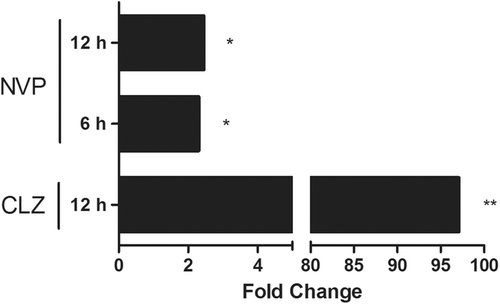
Table 2. Keap1-Nrf2-ARE-regulated genes that were changed ≥1.4 fold by at least one of the aromatic amine drugs (SMX, DDS, or AMG) tested 12 h after treatment.
Figure 7. Hepatic Txnrd activity after treatment with aromatic amine drugs. Thioredoxin reductase activity was increased at both 12 and 24 h after SMX treatment (a) but not with the other two aromatic amine drugs (n = 4; *** p < 0.001). However, a time-course study (b) found that AMG appeared to increase thioredoxin reductase activity 48 h after treatment (n = 2). Rats were treated with 150 mg SMX/kg, 20 mg DDS/kg, or 80 mg AMG/kg before liver samples were taken for activity assay. For timepoints > 24 h, rats were given an additional dose of drug every 24 h.
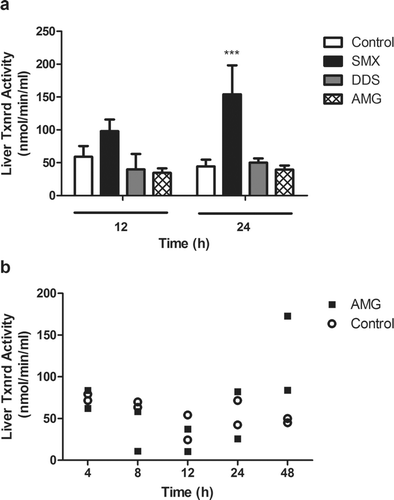
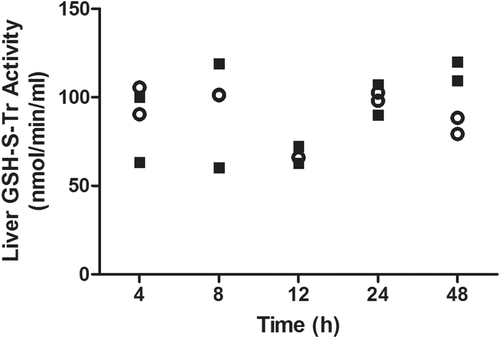
Figure 8. Time-course of hepatic GSH-S-Tr activity in AMG-treated rats. No significant change was observed in GST activity after treatment with 80 mg AMG/kg (solid squares) in the liver as compared to controls (open circles). For timepoints > 24 h, rats were given an additional dose of drug every 24 h (n = 2).
Appendix A. SMX-induced hepatic gene changes at 12 h (fold-change between treated and controls).
Appendix B. DDS-induced hepatic gene changes at 12 h (fold-change between treated and controls).
Appendix C. AMG-induced hepatic gene changes at 12 h (fold-change between treated and controls).