Figures & data
Figure 1. Footpad swelling in naïve juvenile rats following challenge with the C. albicans antigen chitosan. Pre-measurements of the right footpad thickness of naïve juvenile rats (PND 29) were obtained with a digital micrometer prior to challenge with either 75, 150, or 300 µg of chitosan or an equivalent volume of 0.9% saline. The thickness of the right footpad was again measured 24 h later, and the change in footpad thickness for each rat was calculated (post-challenge – pre-challenge thickness). The data are expressed as footpad swelling (mm × 100). Values represent the mean ± SEM from eight animals per group.
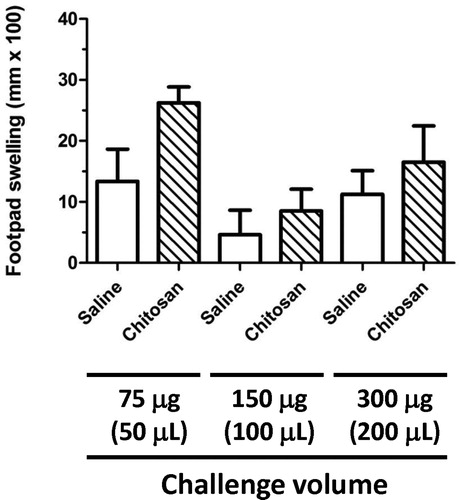
Figure 2. Sensitization timecourse studies for C. albicans in the juvenile rat delayed-type hypersensitivity model. Juvenile rats (PND 28) were sensitized with 2 × 107 C. albicans organisms/rat (200 µl volume) by subcutaneous injection in the right rear flank. On either Day 6, 8, 10, 12 or 14 (PND 34, 36, 38, 40 or 42) pre-measurements of the right rear footpad thickness were obtained with a digital micrometer, and rats subsequently challenged in the right footpad with either 150 µg (100 µl) of chitosan or an equivalent volume of 0.9% saline. The thickness of the right footpad was measured 24 h later and the change in footpad thickness was calculated (post-challenge – pre-challenge thickness). Background footpad swelling was determined in groups of juvenile rats that were challenged but not sensitized (challenge only). Data expressed as footpad swelling (mm × 100). Values represent the mean ± SEM from 10 animals per group. Panels (a) and (b) represent two independent experiments.
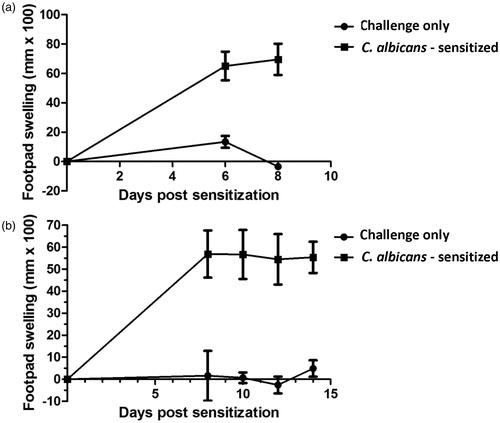
Figure 3. Effect of C. albicans sensitization dose on the footpad swelling response of juvenile rats in the delayed-type hypersensitivity model. Juvenile rats were sensitized on PND 28 with 5 × 106 – 4 × 107 C. albicans organisms per rat (200 µl volume) by sc injection into the right rear flank 10 days prior to challenge. On the day of challenge (PND 38) pre-measurements of the right rear footpad thickness were obtained with a digital micrometer, and rats subsequently challenged in the right footpad by subcutaneous injection of 150 µg (100 µl) of chitosan. The right footpad thickness was measured 24 h later, and the change in footpad thickness calculated (post-challenge – pre-challenge thickness). Background footpad swelling was determined using a group of juvenile rats that were challenged but were not sensitized (challenge only). Data are expressed as footpad swelling (mm × 100) and values represent the mean ± SEM determined from 10 rats per group.
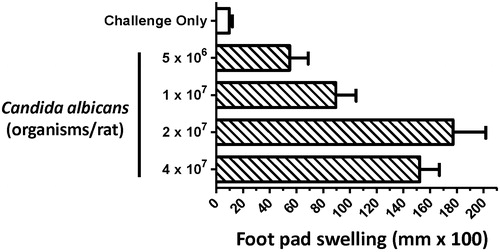
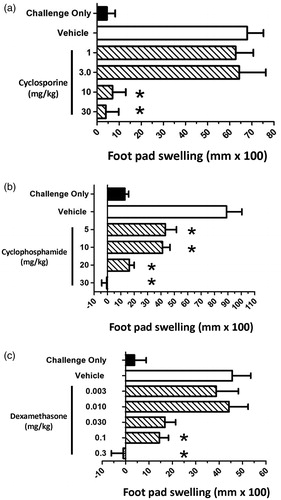
Figure 4. Effect of immunosuppressive drugs on juvenile rat footpad swelling using the C. albicans delayed-type hypersensitivity model. Juvenile rats were administered either vehicle, or (a) CsA, (b) CPS, or (c) DEX at the indicated doses. Dosing began on PND 23, 5 days prior to sensitization, and ended the day prior to challenge (PND 37). Rats were sensitized with 2 × 107 C. albicans organisms/rat by sc injection into the right rear flank on PND 28. On PND 38, pre-challenge footpad thickness measurements were obtained using a digital micrometer, and rats subsequently challenged by subcutaneous injection of 150 µg (100 µl) chitosan into the right footpad. Footpad thickness was again measured 24 h post-challenge and the change in footpad thickness calculated (post-challenge – pre-challenge thickness). Background swelling was determined in groups of juvenile rats that were challenged but not sensitized (challenge only). Data expressed as footpad swelling (mm × 100). Values represent the mean ± SEM from 10 animals per group. *Group mean values statistically different (p < 0.05) from control.