Figures & data
Table 1. List of NK cell activating and inhibitory surface receptors and their ligands.
Table 2. Susceptibility of a number of different stem cells – but not their differentiated counterparts – to NK cell-mediated cytotoxicity.
Figure 1. Immunohistochemical analysis of oral, colon, and breast tumor tissues. (a) Healthy inflammatory oral tissue was stained with antibodies to CD16 (Brown), mast cells (Blue), and Smooth Muscle Actin (SMA) (red). Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) are located below the normal oral epithelium (star), immediately below the basal layer where stem cells and undifferentiated epithelial cells reside. Please note that the epithelial layer is devoid of infiltrating immune cells. (b) Slides from OSCC were prepared and stained with H&E. Infiltration of immune effectors right beneath the epithelial layer can be seen in a connective tissue area where immune inflammatory cells are likely to condition NK cells to lose cytotoxicity and to support differentiation of epithelial cells. (c) Oral tumor tissue stained with the antibodies against CD16, mast cells, and SMA, as indicated above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) are located along oral tumor capsules, with only a few infiltrating immune cells seen within the epithelium (star). (d) Colorectal tumor tissue stained as above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) were located along colorectal epithelial capsules, with only a few infiltrating immune cells seen within the normal (black stars) and tumor (yellow star) epithelium. (e) Breast tumor tissue stained as described above. Immunohistochemical analysis indicated that NK cells (thick arrows) and mast cells (thin arrows) were located along breast tumor capsules, with only a few infiltrating immune cells seen within the epithelium (star).
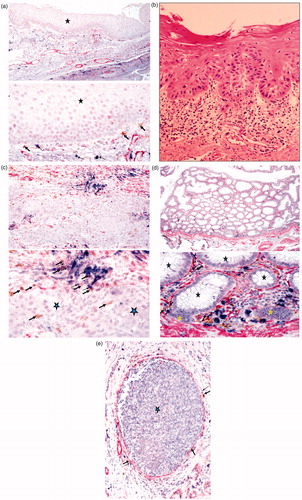
Table 3. Inverse association of target cell susceptibility to NK cell lysis and its ability to inactivate the cytotoxic function of NK cells.
Figure 2. Inverse association between cellular differentiation and NK cell cytotoxicity. Based on the accumulated data from our laboratory, an inverse relationship between cellular differentiation and NK cell mediated cytotoxicity is directly proportional.
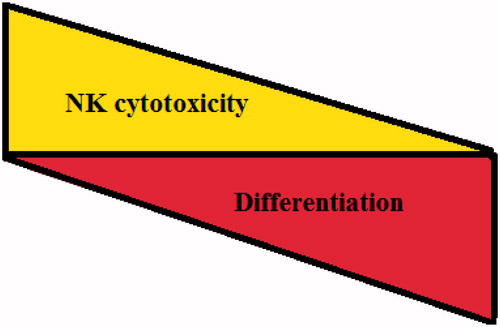
Figure 3. Supernatants from anergized NK cells induced the highest resistance of OSCSC against NK cell-mediated cytotoxicity. Highly-purified NK cells at 106 cells/ml were either left untreated or treated with IL-2 (1000 U/ml), anti-CD16 mAb (3 μg/ml), or a combination of IL-2 and anti-CD16 mAb for 24 h before they were harvested and used to induce differentiation of OSCSC. A total of at 106 OSCSC was added to each plate in 10 ml of media, and the cells were allowed to adhere before NK cell supernatants were added. A total of 180 µl supernatants were added at Days 1, 3, and 5; levels of NK cell cytotoxicity were then determined using freshly-isolated untreated NK cells in a 4-h [51Cr] release assay on Day 6.
![Figure 3. Supernatants from anergized NK cells induced the highest resistance of OSCSC against NK cell-mediated cytotoxicity. Highly-purified NK cells at 106 cells/ml were either left untreated or treated with IL-2 (1000 U/ml), anti-CD16 mAb (3 μg/ml), or a combination of IL-2 and anti-CD16 mAb for 24 h before they were harvested and used to induce differentiation of OSCSC. A total of at 106 OSCSC was added to each plate in 10 ml of media, and the cells were allowed to adhere before NK cell supernatants were added. A total of 180 µl supernatants were added at Days 1, 3, and 5; levels of NK cell cytotoxicity were then determined using freshly-isolated untreated NK cells in a 4-h [51Cr] release assay on Day 6.](/cms/asset/a7caff7b-fab4-408a-a740-d3695671f7a4/iimt_a_877104_f0003_b.jpg)
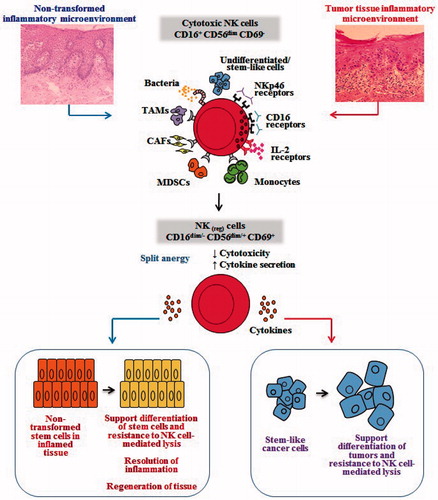
Figure 4. Hypothetical model of induction of conditioned/regulatory NK cells by immune inflammatory cells and by the effectors of connective tissue to support differentiation of the non-transformed stem cells and cancer stem cells. Hypothetical model of NK cell conditioning in tumor microenvironment, as well as in non-transformed immune inflammatory microenvironment, is shown. Significant infiltration of immune effectors right beneath the epithelial layer can be seen in a connective tissue area where immune inflammatory cells are likely to condition NK cells to lose cytotoxicity and gain the ability to secrete cytokines, a term we previously coined ‘split anergy’ in NK cells, and to support differentiation of the basal epithelial layer containing stem cells. NK cells are likely to encounter and interact with other immune effectors such as monocytes/macrophages or other myeloid-derived suppressor cells (MDSC), or with connective tissue-associated fibroblasts (CAF), in order to be conditioned to form regulatory NK (NKreg) cells. NK cells may also directly interact with stem cells at the base of the epithelial layer, in which case by eliminating their bound stem cells, they can become conditioned to support differentiation of other stem cells. In addition, bacteria, through binding to Toll-like receptors can further aid in the generation of NKreg cells. All above-mentioned mechanisms may be operational during inflammatory processes in a tumor microenvironment or in a healthy non-transformed inflammatory microenvironment. NK cell-differentiated epithelial cells will no longer be killed or induce cytokine secretion by the NK cells, resulting in the resolution of inflammation.