Figures & data
Table 1. In vivo techniques that have been commonly used to assess the digestion and absorption of conventional chemicals in the gastrointestinal tract, and could be relevant to the assessment of NMs.
Table 2. Ex vivo tissue models that have been commonly used to assess the absorption of conventional chemicals through the gastrointestinal tract, and that could be relevant to the assessment of NMs.
Table 3. In vitro cell culture models that simulate the gastrointestinal tract epithelium, are commonly used to assess the absorption of conventional chemicals, and could be relevant for the assessment of NMs.
Table 4. In vitro non-cellular fluid models that simulate the gastrointestinal tract, are commonly used to assess the digestion and absorption of conventional chemicals, and could be relevant to the assessment of NMs.
Table 5. In silico computational models that simulate the gastrointestinal tract, are increasingly used to assess the dissolution and absorption of conventional chemicals, and could be relevant to the assessment of NMs.
Figure 1. Control treatments for in vivo, ex vivo and in vitro nanomaterial digestion and absorption assays. (A) Background levels of the nanomaterial in the model should be measured in a control well receiving the vehicle components. (B) The controls are compared with the nanomaterial treatment group. (C) If a fraction of the nanomaterial surface dissolved in simulated gastrointestinal fluids, that concentration of ions can be administered alone, to determine if the ions are responsible for any absorption. (D) If the label dissociated from the nanomaterials in simulated gastrointestinal fluids, that concentration of label can be administered alone, to control for false positives from its absorption.
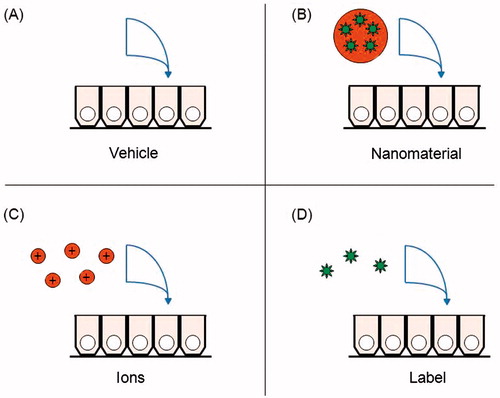
Figure 2. Categories of gastrointestinal tract models that demonstrate potential utility for the assessment of the digestion and absorption of engineered nanomaterials released from food. Specific examples are provided of models that have been used, or could be further developed, to quantify digestion and absorption parameters relevant to nanomaterials. The general parameters that the models could measure are listed.


