Figures & data
Figure 1. A. A well-defined 11 × 9 × 8 cm expansile osteolytic lesion extending from the left ischium to the lower arm of the left pubis in a 52-year-old man with progressive pain in his left hip and leg. Radiographic findings were consistent with the diagnosis of GCTB. B. 3 months after the start of denosumab treatment, there was no progression of disease and increased radiopacity of the lesion. C. 5 years after resection of the ischium and inferior ramus of the pubis followed by phenolization and cementation of the posterior acetabular wall, there was no evidence of recurrent disease.
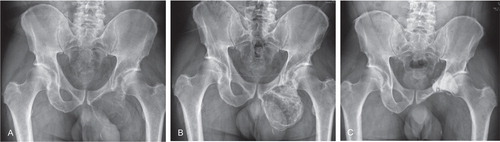
Figure 2. A. T1-weighted MR imaging before treatment with denosumab showed a highly vascularized lesion (arrowheads) extending into the surrounding soft tissues (arrow), consistent with GCTB. B. T1-weighted MR imaging 3 months after the start of denosumab treatment showed that there was no progression of disease. Areas of central necrosis were seen (arrowheads).
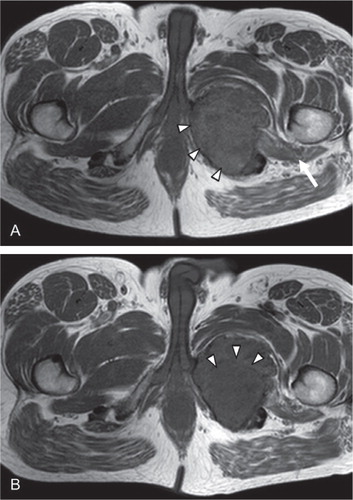
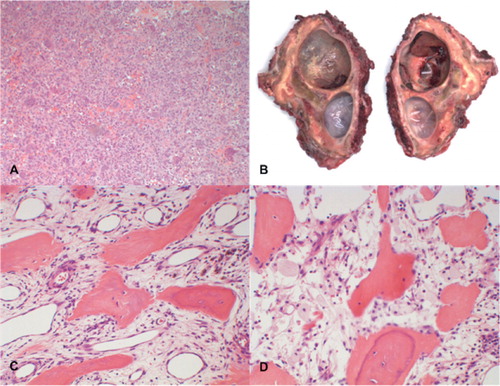
Figure 3. A. Histology of the biopsy specimen before the start of denosumab treatment showed numerous uniformly spaced giant cells with large vesicular nuclei, prominent nucleoli, and mononuclear cells with eosinophilic cytoplasm and nuclei similar to those of adjacent giant cells, which is consistent with the diagnosis of GCTB. B. Macro specimen of intralesional resection after 7 months of treatment with denosumab, showing 2 large cysts and focal clots. The resection planes were not free of reactive tissue. C and D. Histology of the surgical specimen showed stromal cells, scattered mononuclear spindle cells with elongated oval-shaped nuclei without evident atypia, and diffuse foamy macrophages. No multinucleated giant cells were seen.