Figures & data
Table 1. Patient characteristics and clinical details
Figure 1. The CAD/CAM shoulder (Stanmore Implants, Elstree, UK). A custom-made, constrained hip arthroplasty-inspired implant. The implant comprises an uncemented titanium glenoid shell mated to a cobalt-chrome tapered humeral stem (cemented or uncemented with a 28-mm or 32-mm head) with a high-molecular-weight polyethylene liner (cemented into the glenoid shell). A. A preoperative drawing. B. The actual implant.
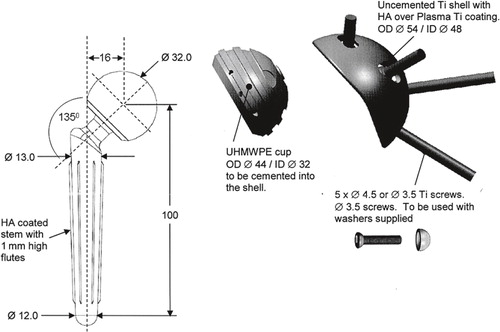
Figure 2. A 72-year-old man (patient 6) presented with severe pain and limited function 6 years after reverse shoulder arthroplasty for primary cuff-tear arthropathy. Shoulder radiographs at presentation showed loosening and migration of the baseplate (panel A). The prosthesis was revised using the CAD/CAM shoulder with significant pain relief and improvement in functional scores at the 36-month follow-up (panels B and C).
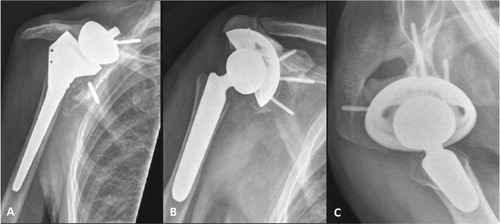
Table 2. Summary of outcome measures before and after revision
