Figures & data
Table 1. Composition and size of liposome prepared using soy lecithin and cholesterol in various ratios.
Figure 1. Post-thaw motility in normozoospermic ejaculates cryopreserved with glycerol egg yolk citrate (GEYC) buffered and liposome based freezing medium (LFM). The spermatozoa were analyzed for the motility after thawing. The data from 28 subjects represented as the mean motility and the error bars represent the standard error of mean (SEM) value. Liposome based medium was prepared with liposomes encapsulated with soy lecithin and cholesterol at various ratios and designated as LFM1 (soy lecithin: cholesterol: 95:5), LFM2 (soy lecithin: cholesterol: 90:10), LFM3 (soy lecithin: cholesterol: 80:20), and LFM4 (soy lecithin: cholesterol: 70:30). The freeze-thaw process resulted in a significant reduction in total and progressive motility in post-thaw samples of GEYC, LFM1, LFM2, and LFM4 groups compared to that of the neat sample (#p < 0.001). Similarly, the total motility and progressive motility was higher in samples cryopreserved with liposome based medium compared to those cryopreserved with GEYC medium (**p < 0.01, ***p < 0.001). ▪: total motility; ▪: progressive motility.
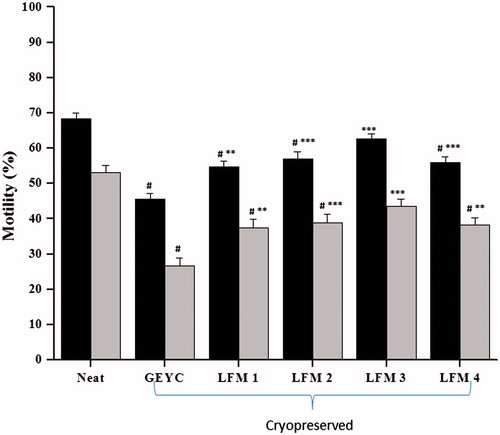
Figure 2. Sperm yield by swim up technique in normozoospermic ejaculates cryopreserved with glycerol egg yolk citrate (GEYC) buffered and liposome based freezing medium (LFM). The liquefied semen samples from 28 normozoospermic subjects were divided into five equal parts after the analysis and cryopreserved with GEYC or LFM based cryopreservation medium. The LFM was prepared with liposomes encapsulated with soy lecithin and cholesterol at various ratios and designated as LFM1 (soy lecithin: cholesterol: 95:5), LFM2 (soy lecithin: cholesterol: 90:10), LFM3 (soy lecithin: cholesterol: 80:20), and LFM4 (soy lecithin: cholesterol: 70:30). The semen samples were thawed and the motile sperm were extracted by swim up technique. After 1 hour of incubation, the overlay was collected from which the sperm density was assessed. The data represents the mean sperm density in millions per ml and standard error of mean (SEM) value. Even though the sperm yield was higher in the LFM2 and LFM3 groups compared to the GEYC group, the difference was statistically non-significant (p > 0.05).
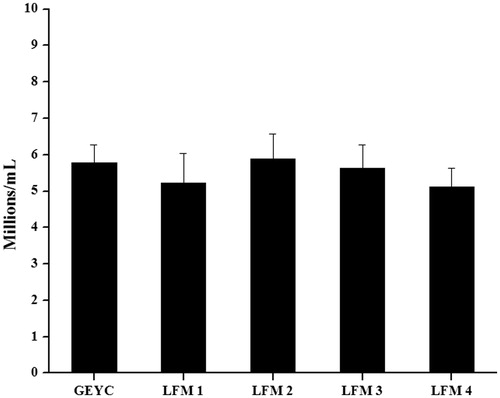
Table 2. Progressive motility in human spermatozoa subjected to freeze-thaw process using cryopreservation medium with liposomes of soy lecithin and cholesterol in various ratios.
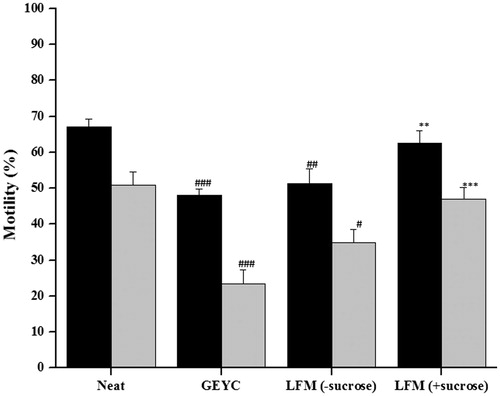
Figure 3. Sperm motility in normozoospermic ejaculates frozen with liposome based cryopreservation medium (LFM) with or without sucrose. The semen samples from 10 normozoospermic subjects were divided into three parts. Semen samples were cryopreserved with glycerol egg yolk citrate (GEYC) medium, LFM3 medium (soy lecithin: cholesterol: 80:20) with sucrose (10% w/w) and LFM3 medium (soy lecithin: cholesterol: 80:20) without sucrose. The data represents the mean motility and standard error of mean (SEM). Both total and progressive motility was significantly lower in semen samples cryopreserved with conventional GEYC medium and LFM without sucrose (#p < 0.05, ##p < 0.01, ###p < 0.001). However, LFM with sucrose had a significantly higher percentage of total (**p < 0.01) and progressive motility (***p < 0.001) compared to samples cryopreserved in GEYC. ▪: total motility; ▪: progressive motility.