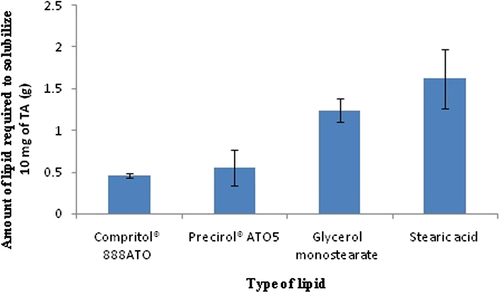
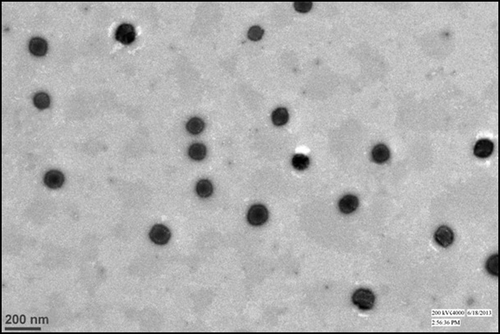
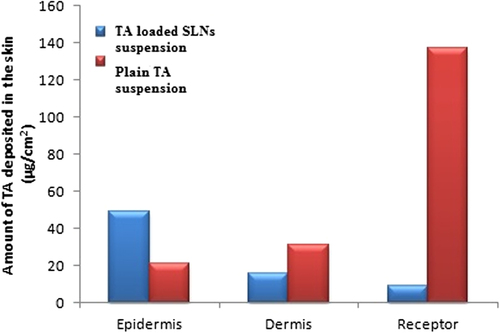
Figures & data
Table I. Formulation design for SLNs.
Table II. Size, PDI, ZP, EE, and L of the drug loaded solid lipid nanoparticles (data represent mean ± SD).
Figure 2. (a) Mean particle size and polydispersity index of selected batch formulation; (b) Zeta potential of the formulation of the selected batch.
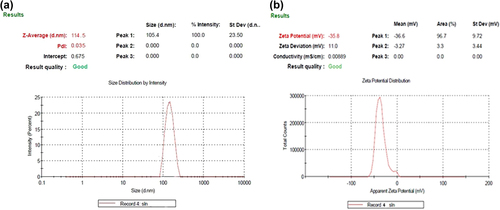
Figure 4. XRD analysis. (a) Pure TA (b) Compritol® 888ATO (c) Physical mixture of TA and Compritol® 888ATO (d) TA loaded SLNs.
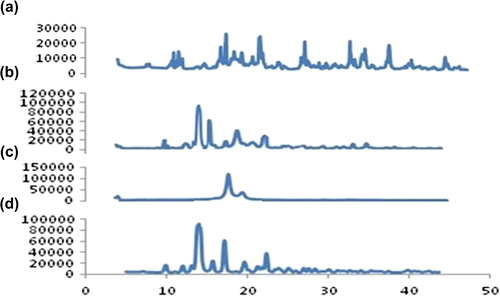
Figure 5. DSC thermograms. (a) Pure Compritol 888 (b) Pure TA (c) Physical mixture of TA and Compritol 888 ATO (d) TA loaded SLNs.
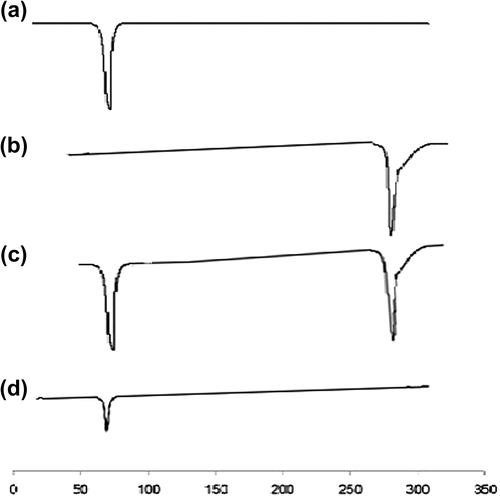
Figure 6. Drug release profile from pure TA suspension and drug loaded SLNs suspension in phosphate buffer (7.4pH).
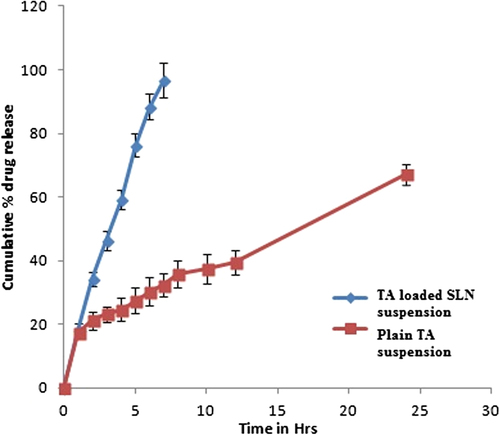
Table III. Stability study of selected formulation.