Figures & data
Table I. Description of immunization protocol.
Figure 4. IgG titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
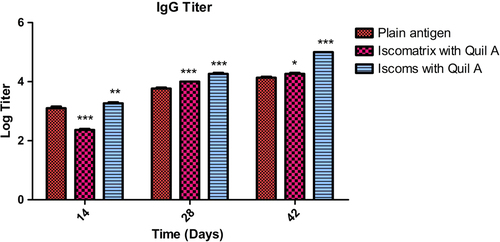
Figure 6. IgM titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
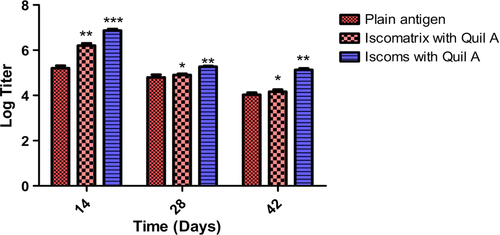
Figure 7. IgG1 titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.
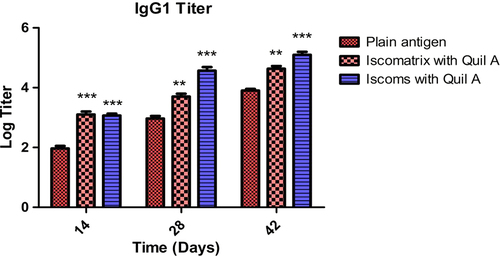
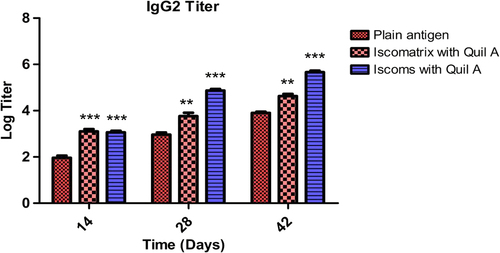
Figure 8. IgG2a titer after 14, 28 and 42 days following pulmonary immunization. Statistical analysis was carried out by two-way analysis of variance followed by post-hoc Bonferroni post-tests comparing all data vs. control. Data compared with plain antigen as control. ‘*’ denotes P < 0.05, considered as significant, ‘**’ denotes P < 0.01 and ‘***’ denotes P < 0.001.